Home > Health & Education > eAdvances
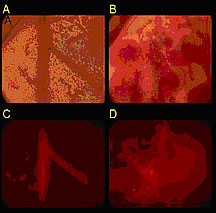
Targeted Drug Delivery With Microbubbles: September 16, 2005Treating malignant brain tumors can be difficult. The protective blood-brain barrier blocks many drugs from acting on brain cells. Now, a new method for delivering drugs directly to the affected area may increase the effectiveness of chemotherapy in brain tumors and reduce its toxic effect on healthy cells.Researchers at the University of California, Davis, and ImaRx Therapeutics in Tucson, Arizona, are developing an innovative technique that uses ultrasound and drug-laden “microbubbles” to deliver concentrated chemotherapy drugs to the inner lining of blood vessels. Doctors already use ultrasound to identify tumors and guide biopsy procedures. Ultrasound pulse sequences can also guide micro-packaged medications to specific parts of the body. Ultrasound as a GuideNIBIB grantee Dr. Katherine Ferrara, professor of biomedical engineering at the University of California, Davis, and her student Michaelann Shortencarier have shown that ultrasound can guide tiny gas bubbles filled with fluorescent dye to a particular site, and then bursts of ultrasound can fragment the bubbles and spray their contents onto diseased tissue. Pre-clinical studies are under way. Ferrara , Shortencarier, and colleagues use acoustically active lipospheres (AALs) —microscopic bubbles with a gas center that’s surrounded by a thick oil shell and encased by an outer layer of fatty substances known as lipids. The outer lipid coating of the microbubble encloses the drug while it circulates throughout the body, preventing systemic toxicity while providing concentrated drug delivery to a specific region. In their experiments, the researchers filled the lipospheres with a fluorescent dye and guided them to a target site using a specially devised pattern of ultrasound pulses. Applying ultrasound to the lipospheres causes the gas bubbles inside them to expand and contract. A series of ultrasound pulses is tailored specifically to increase the close contact between the lipospheres and their target. Once the lipospheres are in place, a high-intensity ultrasound pulse breaks the bubbles into tiny fragments, releasing the dye. The researchers are currently focusing on delivering the lipospheres to blood vessel walls, because blood vessels provide nourishment to tumors. From Lab to ClinicThe liposphere/ultrasound approach offers several advantages over existing drug delivery methods. Ultrasound can selectively deliver drug-laden lipospheres to a tumor or organ and then release the drug at a particular site. The force used to propel the drug ensures that it is delivered specifically to the endothelial or surface cells of blood vessels in the target area and not simply released to flow downstream. “The idea is that you can use ultrasound pressure to prevent the drug delivery vehicle from being uniformly distributed throughout the body,” Dr. Ferrara says. Ferrara hopes this technology will aid in managing brain cancer. To be beneficial, drugs used to treat brain tumors must cross the blood-brain barrier. However, this protective defense blocks the therapeutic action of many drugs and often renders chemotherapy ineffective. When the barrier is crossed, the entire brain, not just the affected site, is subject to the toxic effects of chemotherapy. Although human clinical trials are still several years away, Ferrara says that delivering drugs via ultrasound may produce fewer side effects and improve patient outcomes. The researchers’ goal is to have the pulse patterns used for guiding and then rupturing microbubbles integrated into conventional ultrasound machines, thereby linking diagnostic and therapeutic work. The team wants to develop a wider range of pulse sequences and drug-delivery vehicles to treat other diseases as well. ”We’re exploring the use of these techniques in a broad range of clinical applications,” says Ferrera. Funding for the research was provided by the National Institute of Biomedical Imaging and Bioengineering and the National Cancer Institute. Reference: Shortencarier M, et al., A method for radiation-force localized drug delivery using gas-filled lipospheres, IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 51, 821-830, 2004. |
 |
 |
Department of Health and Human Services |
 |
National Institutes of Health |
 |