VRC Boosts Research on Vaccines for HIV and Other Diseases
The global AIDS epidemic is one of the most significant infectious disease threats to human health. Although AIDS deaths have fallen significantly in many developed countries, the disease continues to accelerate in the developing world. NIAID’s Vaccine Research Center (VRC) was conceived in response to this growing epidemic.
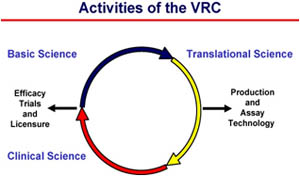 |
| The approach of the VRC in basic, translational, and clinical research. Credit: NIAID |
The VRC is a specialized research program within NIAID. It is structured to facilitate a multidisciplinary effort in moving science discoveries from the lab into the clinic and brings together basic scientists, clinical investigators, and translational researchers to accelerate the development of novel vaccine products. Initial VRC goals included the design and development of effective vaccine candidates, with an emphasis on HIV/AIDS; the evaluation and optimization of the immune responses generated by these candidates; and the advancement of the most promising candidates into clinical trials.
Using Recombinant DNA in Vaccine Design
In the process of designing candidate vaccines, the VRC has developed innovative recombinant gene technologies. Recombinant DNA (rDNA) is DNA that has been artificially created. DNA from two or more sources is combined into a single, “recombinant,” molecule. The host cells takes up the DNA, express the antigen (the substance that stimulates an immune response), and present it to the immune system in a manner similar to natural infection.
DNA vaccines rely on a ring of nucleic acid, called a plasmid, instead of a weakened or inactivated virus, to encode an antigen that elicits an immune response strong enough to protect against disease. Such plasmids can hold multiple genes for multiple antigens, and rDNA technology offers an easy way to mix and match antigens in new, efficient combinations.
VRC programs have also applied atomic-level, structural knowledge of HIV to improve understanding of how HIV evades the immune system and to design novel antigens. VRC scientists have screened many different gene constructs in order to optimize the antigens that stimulate potent immune responses.
Expanding its Program
Over the last several years, the VRC research program has expanded to include vaccine development for Ebola virus, Marburg virus, influenza, severe acute respiratory syndrome (SARS), and West Nile virus, as well as development of improved vaccines for smallpox. Of these new programs, the largest research and development effort is directed toward a vaccine to protect against Ebola and Marburg viruses.
For influenza, the VRC is developing and testing gene-based and protein-based vaccines to protect against multiple avian flu strains. Modifying the structure of existing flu proteins can guide the development of potential vaccines and therapeutics that can be evaluated before a human strain of avian flu actually arises. Because most vaccines, like the seasonal flu vaccine you probably received this winter, can be developed only after a strain emerges, the protein-based vaccine would give vaccine developers a head start, which could save valuable time and many lives in the event that a human strain of avian flu does emerge.
Advancing Technology
The efforts of VRC research scientists are facilitated by core laboratories in immunology, flow cytometry, and vector development; a biocontainment laboratory; an animal facility; and a clinical trials program. The VRC has also created specialized capabilities in structural biology, especially crystallography, and has developed state-of-the-art methods in bioinformatics for vaccine development.
In addition, the VRC has developed and implemented a vaccine production plant in Frederick, MD. This 126,900 square-foot production facility can produce sufficient clinical vaccine material to conduct several Phase I clinical studies per year and has the flexibility to meet material needs for advanced clinical studies.
The production facility complies with current good manufacturing practices (cGMP) and incorporates numerous features to allow flexibility in processing multiple products simultaneously while preventing cross contamination. The rapid transition of SARS and H5 (avian) influenza DNA vaccine products from the VRC laboratories through cGMP manufacture and into Phase I studies would not have been possible without this vaccine manufacturing capability.
Moving Vaccines Through Production and Beyond
The VRC has also established the NIAID Vaccine Immune T-Cell and Antibody Laboratory (NVITAL) to develop immune assays, which measure and characterize human responses to NIAID candidate vaccines used in clinical studies. The Food and Drug Administration (FDA) requires vaccine manufacturers to perform key immune assays according to FDA guidelines before it will license candidate vaccines. The NVITAL facility fills this urgent need for NIAID-sponsored products progressing through clinical trials.
The close collaboration between VRC core facilities and VRC research laboratories has been a critical factor in the VRC’s ability to accelerate vaccine research and development. VRC scientists have expertise across the spectrum of the vaccine development process: basic immunology, molecular biology, preclinical testing, clinical trials, and vaccine production. These areas complement each other and promote a spirit of collaboration and exchange, both within the center and with outside collaborators.
back to top