National Clinical Trials Program in Treatment and Prevention
Goal
The Challenge
Progress Toward Meeting the Challenge
2003 Plan and Budget Increase Request

Goal
Ensure that clinical trials address the most important questions in treatment and prevention and are broadly accessible.

The Challenge
Clinical trials are the final, definitive step in testing new approaches to cancer prevention, diagnosis, and treatment. Our National Clinical Trials Program essentially is a laboratory without walls, through which NCI has a tangible and direct impact on the survival and quality of life of patients with cancer.
NCI's clinical trials system is complex, involves many participants, and requires collaboration at all levels, among investigators and physicians, industry and academia, academia and NCI, and NCI and industry.
Adding to this complexity, cancer clinical trials have undergone a number of dramatic changes in recent years.
- Progress in cancer biology, genetics, immunology, molecular biology, and imaging technology has accelerated, creating new opportunities to improve clinical practice.
- As cancer researchers around the country have identified the molecular changes that cause a normal cell to become cancerous, the number of new anti-cancer agents that target these changes has rapidly grown, triggering an entirely new approach to the development of cancer drugs and a rapid growth in NCI-sponsored clinical trials for treating and preventing cancer.
- At the same time, advances in informatics and electronic communications have led to new approaches to communication and data reporting and analysis in the clinical research setting, providing new opportunities to enhance the efficiency of clinical trials and speed their results to the care of cancer patients. NCI's cooperative groups - networks of investigators who conduct clinical trials - have begun to incorporate many of these advances into their clinical trials, but much work remains to be done.
Top of Page

Despite progress made to date, many barriers to clinical trials participation persist.
In particular, the reimbursement that NCI provides physicians for their role in clinical trials often falls far short of their costs and is well below what the pharmaceutical industry provides. Physicians who take part in clinical trials often must hire additional nursing and data management staff to ensure that patients fully understand the risks and benefits of participation, track participating patients, and collect and report the necessary data.
A 1999 survey of oncologists by the American Society of Clinical Oncology found that although many physicians preferred NCI-sponsored cooperative group clinical trials, inadequate reimbursement for the costs and time required for data reporting were barriers to participation.
While NCI has improved the availability of clinical trials information to patients and health care professionals (see the Extraordinary Opportunity in Cancer Communications) and has doubled its reimbursement over the past several years, it still lags far behind actual costs and industry standards. This problem is compounded in cancer prevention trials because many thousands of patients often are needed to evaluate preventive measures and these patients must be followed for longer periods of time.
NCI's challenge is to ensure that we overcome the barriers to participation in clinical trials and that we capitalize on the latest developments in cancer research, informatics, and management to address our most important questions in cancer prevention and treatment.
Top of Page

Progress Toward Meeting the Challenge
Increasing the Efficiency of Clinical Trials
Recent Clinical Trial Results
An Increasing Focus on Targeted Therapies
Cancer Prevention Trials

Increasing the Efficiency of Clinical Trials
- In our ongoing efforts to improve the speed and efficiency with which cancer clinical trials are conducted, NCI recently centralized the common administrative, financial, and data collection activities of its clinical trials cooperative groups. Through an online Cancer Trials Support Unit site unveiled in 2000, cooperative group investigators can now:
- Download clinical trial protocols and other information.
- Enroll patients in clinical trials.
- Arrange for reimbursement of research costs.
- Receive alerts when new trials begin.
In 2002, oncologists outside the cooperative groups also will be permitted to enroll their patients in these clinical trials.
- Similarly, the four cooperative groups conducting studies on cancer in children merged into a single new Children's Oncology Group. This consolidation is expected to:
- Potentially double the number of doctors and hospitals involved in any given study.
- Allow trials to be completed more quickly.
With cure rates for new childhood cancers now reaching 70 percent, the new cooperative group will be able to devote more of its energies to the less common childhood cancers, for which cures have not been as forthcoming.
Top of Page

Recent Clinical Trial Results
Over the past two years, clinical trials have continued to contribute to improvements in survival and quality of life for patients with a wide variety of cancers. For example, recently completed clinical trials have determined that:
- Cervical cancer, still the second leading cause of cancer death in women around the world, can be treated more effectively by combining cisplatin chemotherapy with radiation treatment. It is estimated that this treatment can save 2,000 additional lives each year in the United States and considerably more - perhaps hundreds of thousands - worldwide.
- The combination of chemotherapy and radiation following surgery substantially prolongs the survival of patients with stomach cancer.
- For patients with metastatic kidney cancer, surgery to remove the kidney can add months to patients' lives.
- Preoperative chemotherapy prolongs the survival of patients undergoing bladder cancer surgery.
- In the most aggressive cases of prostate cancer, radiation therapy combined with the optimal application of hormone treatments leads to longer survival.
- The use of cyclooxygenase-2 (COX-2) inhibitors reduces the number of colon polyps in patients with the genetic disorder Familial Adenomatous Polyposis. Without treatment, patients with this condition typically develop numerous polyps, increasing their risk for colon cancer.
Top of Page

An Increasing Focus on Targeted Therapies
The recent improvements in cancer therapy described above generally combine advances in conventional anti-cancer treatments with surgery, chemotherapy, or radiation. But the past decade also has seen an explosion in our understanding of tumor biology and immunology, which has led to the identification of a vast array of new molecular targets at which to direct treatment and prevention interventions. The cellular pathways and interactions involved in these molecular targets are extraordinarily complex and inter-related, and they require scientists to develop new techniques and tests to identify patients whose tumors contain the relevant targets.
As a result, clinical trials of targeted agents often involve laboratory studies to better define the presence of targets and drug effects in the tumors of individual patients undergoing treatment. Indeed, more than half the cancer treatment trials initiated by NCI's cooperative groups over the last two years have included correlative studies. This trend is also increasingly seen in cancer prevention trials involving chemopreventive agents.
Along with the discovery of more and more therapeutic targets for cancer, there has been a huge increase in the number of new anti-cancer agents in drug development. According to the Pharmaceutical Research and Manufacturers of America, more than 400 anti-cancer agents were in development in 2001, up from fewer than 100 in the late 1980s. Similarly, the number of pharmaceutical and biotechnology companies developing anti-cancer agents nearly quadrupled over the same period, rising from 45 to 170.
Despite this large increase in corporate involvement, NCI's role and the public-private partnerships it brokers continue to be essential. NCI collaborates with industry in the development of many promising investigational agents for treatment and prevention by sponsoring clinical trials for them.
Because pharmaceutical companies tend to seek FDA approval or licensing of a new agent only for a single tumor type, NCI's involvement in the drug development process helps ensure that new agents are evaluated against the full range of cancers for which they may be effective and in combination with treatments such as surgery and radiation therapy.
These collaborations bring new treatments and prevention strategies to patients years earlier than would otherwise occur. The figure below illustrates the rapid expansion of clinical trials in many different tumors, made possible by the collaboration between Novartis Pharmaceuticals and NCI-supported researchers to develop Gleevec™.
More on the development of Gleevec
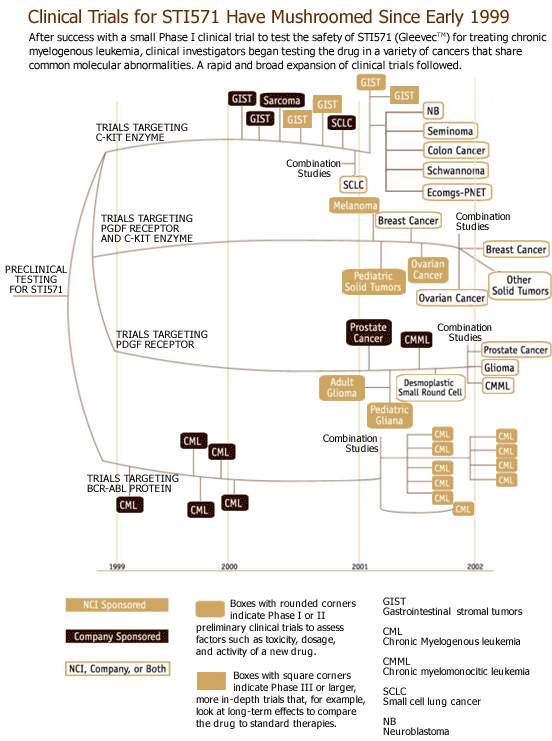
Top of Page

Cancer Prevention Trials
As scientists discover more and more about the basic mechanisms of cancer, they also add to our knowledge about its prevention, allowing experts to explore the possibility of averting cancer through chemoprevention. Unlike conventional approaches to preventing cancer, which often focus on avoiding exposure to cancer-causing agents (such as tobacco and excessive sunlight), chemoprevention actively intervenes against cancer with drugs or other agents that stop the transformation of normal cells into cancer cells.
- One of the most widely used chemopreventive agents today is tamoxifen, a drug that interferes with the activity of estrogen and was initially introduced as a treatment for breast cancer. After physicians began to report that women who had received tamoxifen following breast cancer surgery were less likely to develop cancer in their other breast, NCI initiated the first large breast cancer prevention trial in the United States to determine whether the use of tamoxifen could prevent breast cancer in high-risk women.
Initial results from that trial, announced in 1998, indicated that tamoxifen did indeed reduce the risk for breast cancer, and that its use could be especially beneficial for young women at significant risk for the disease.
But because tamoxifen also carries potentially serious risks, such as blood clots and stroke, NCI continues to sponsor other clinical trials in breast cancer prevention. A major NCI-supported study is comparing tamoxifen with raloxifene, an osteoporosis prevention drug that also appears to lower the risk for breast cancer.
- A number of other NCI-sponsored studies are examining the potential for the arthritis drug celecoxib (Celebrex™) to prevent colon and other cancers. For arthritis sufferers, celecoxib reduces inflammation and alleviates symptoms by inhibiting the body's production of COX-2 enzymes. Researchers suspect that celecoxib might play a valuable role in cancer prevention because precancerous tissues, such as colon polyps, also produce COX-2 enzymes, and because epidemiologic studies have shown that arthritis sufferers who regularly use anti-inflammatory drugs have lower rates of colon cancer.
In NCI-sponsored studies thus far, celecoxib has been found to reduce the number of colon polyps in patients with Familial Adenomatous Polyposis, an inherited syndrome that predisposes them to colon cancer. Other NCI-funded clinical trials are investigating whether celecoxib can prevent esophageal, bladder, and skin cancers.
More information on NCI-Sponsored Trials of Celecoxib
Top of Page

The Plan - National Clinical Trials Program
Goal
Ensure that NCI's clinical trials program is poised to address the most important medical and scientific questions in cancer prevention and treatment quickly and effectively through state-of-the-art clinical trials that are broadly accessible to cancer patients, populations at risk for cancer, and the physicians who care for them.
Fiscal Year 2003 Objectives, Milestones, and Funding Increases Needed
Top of Page

|
Objective 1: Identify and address compelling clinical questions confronting physicians and their patients struggling with cancer or at high risk of cancer. |
- Expand clinical trials planning so that critical treatment and prevention questions are addressed across the major types of conditions experiences by patients.
| $1.00 M |
- Expand State of the Science meetings to cancers beyond gastrointestinal, lung, genitourinary, and leukemia to identify important research questions and define a scientific research agenda to address them.
| $1.00 M |
- Provide additional research funds for scientific leadership support for researchers who chair studies in addition to caring for patients and for statisticians; together they are responsible for writing, monitoring, and analyzing NCI-sponsored, high-priority Phase III trials.
| $3.00 M |
- Increase translational research funds for clinical correlative studies to uncover the mechanisms of action, response, and resistance underlying new treatments and preventive strategies and to translate basic biology from the laboratory to clinical practice.
| $8.00 M |
- Support a national tissue resource system that includes normal, precancerous, and cancer tissues to facilitate rapid evaluation of new assays and relevant clinical correlations as new targets are identified. (Budgeted in Defining the Signatures of Cancer Cells, objective 2)
|  |
- Fund tissue and specimen banks to store material from cancer patients undergoing treatment and from those at risk of developing cancer to allow later studies of drug effectiveness, molecular abnormalities, and clues to tumor initiation and progression.
| $4.00 M |
|  |
| TOTAL | $17.0 M |
Top of Page
| Objective 2: Enhance the ability and flexibility of the clinical trials system to respond quickly and effectively to scientific opportunities emerging from the vast expansion of molecular targets discovery, new drug discovery, and translational research. |
- Create flexible collaborations among investigators to facilitate multi-institutional clinical trials, projects, and consortia.
| $5.00 M |
- Integrate scientific strategic planning to include cross-disciplinary input (e.g., oncology and diagnostic imaging) and project teams.
|  |
- Incorporate other relevant research questions into treatment and prevention trials, and utilize the clinical trials infrastructure more broadly.
|  |
- Incorporate behavioral, epidemiologic, outcomes, and other relevant research to effectively address cancer questions in specific tumor types and patient populations.
| $0.50 M |
- Incorporate the evaluation of relevant biomarkers into clinical trials.
|  |
| TOTAL | $5.5 M |
Top of Page
| Objective 3: Increase the pace of development and clinical testing of promising new therapeutic and preventive agents. |
- Over 2 to 3 years, substantially increase the number of promising agents entering NCI-sponsored clinical trials, triple annual patient accrual to early clinical trials of promising agents, and substantially increase the number of pivotal or proof-of-principle early clinical trials.
|  |
|  |
- Increase funding for early treatment and prevention agent development and for Interdisciplinary Research Teams in Interventions Directed at Molecular Targets to develop the necessary assays, tools, and approaches to assess the effects of promising new agents on their molecular targets.
| $20.00 M |
- Support a rapid grant review process for mechanism-based clinical trials.
|  |
- Support the infrastructure needs of the intramural clinical trials program at the NIH Clinical Center by increasing the numbers of data managers, research nurses, biostatisticians, and clinicians to support a critical mass of clinical investigators; continue to develop the net-Trials database system to link all NCI intramural clinical investigations and to serve as a prototype that other institutions can use; and continue the Tissue Array Research Program to identify key molecular alterations in cancers.
| $15.00 M |
| TOTAL | $35.0 M |
Top of Page
| Objective 4: Double the rate at which Phase III trials are completed. |
- Shorten the duration of patient accrual to important national trials (approximately 5 years at present) by 50 percent, thereby substantially increasing the number of new treatments or interventions that can be definitively evaluated.
|  |
- Provide funds to adequately support data management and enable substantially greater physician and patient participation in clinical trials:
- Increase the reimbursement to $3,500 per patient for treatment and prevention trials to adequately cover the additional nursing and data management costs required to participate in clinical trials.
- Double the number of patients accrued to treatment and prevention trials over 1 to 2 years.
- Provide follow-up funding to allow physicians to follow patients and report outcome data for many years and address important long-term treatment and epidemiology issues.
| $350.0 M |
- Expand the Clinical Trials Support Unit to consolidate the administrative tasks associated with clinical trials and to provide a single interface for investigators enrolling patients.
| $20.00 M |
- Provide extensive information about prevention and treatment options and clinical trials to enable patients and physicians to make informed medical choices. (Budgeted in Cancer Communications, Objective 2)
|  |
|  |
| TOTAL | $370.0 M |
Top of Page
| Objective 5: Reduce outcome disparities in special populations by increasing access to state-of-the-art clinical trials in cancer prevention and treatment. |
|  |
See Understanding Clinical Trials.
Top of Page
|



