
The Mathematics of Cancer
To most people, soil nematodes - microscopic parasites critical to the breakdown of organic matter in soil - would appear to have no relation to cancer research. But Drs. Vito Quaranta and Alexander "Sandy" Anderson might beg to differ. A collaboration between the two to develop complex mathematical models that drive computer simulations of tumor invasion began with adaptation of a model developed by Dr. Anderson designed to predict soil nematode migration.
"Mathematicians have been doing cancer modeling for 50 years," says Dr. Quaranta, director of the Vanderbilt University Integrative Cancer Biology Center. But it's only in the current decade, he adds, that cancer biologists have connected with a new generation of mathematicians like Dr. Anderson, from the University of Dundee in Scotland, to develop mathematical models intended to capture and integrate the complex factors involved in cancer development, progression, and metastasis.
This in silico movement in cancer research truly is in its nascent stages. Only a handful of laboratory and clinical cancer researchers are seriously collaborating with biological mathematicians, and in silico studies are only now breaching the upper echelons of oncology journals.
But according to Dr. Daniel Gallahan, head of NCI's Integrative Cancer Biology Program, which is supporting the development of in silico cancer research, the time and need for it have arrived.
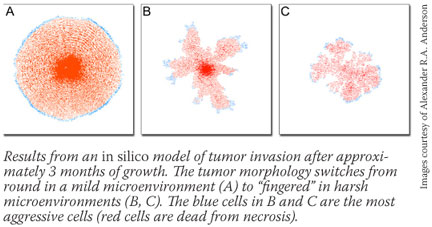 Cancer researchers have done a remarkable job of discovering and characterizing the important biological and molecular parts of the cancer process, Dr. Gallahan stresses, including genes, intracellular signaling pathways, and, more recently, microRNA. Cancer researchers have done a remarkable job of discovering and characterizing the important biological and molecular parts of the cancer process, Dr. Gallahan stresses, including genes, intracellular signaling pathways, and, more recently, microRNA.
"But once you start trying to assemble all of those pieces, that's when it becomes daunting," Dr. Gallahan says. "That's when it becomes a real challenge for human understanding and intuitiveness to look at a situation and extrapolate what is actually happening."
Computational modeling, he continues, can help to develop a fuller picture of cancer as a complex biological system, and help better assess what factors within and around a given tumor decide its fate. Evidence to support that belief is mounting. In silico studies published over the past few years, for instance, have highlighted the tumor microenvironment's potentially critical influence on tumors.
These studies include an October 2005 study published in Clinical Cancer Research by Dr. Vittorio Cristini and a team from the University of California, Irvine, that described computational simulations of brain tumors suggesting that a tumor's aggressiveness, as indicated by its shape, or morphology, was greatly influenced by factors such as the amount of oxygen in its environment. Sufficiently oxygenated microenvironments led to spherically shaped, localized tumors, while oxygen-choked surroundings, including those created by simulated anti-angiogenic treatment, generated tumors that snaked out into nearby tissue.
And in December 2006, Drs. Quaranta and Anderson, building on a paper published in March 2005 in a mathematics journal, published computational simulations in Cell predicting that a harsh tumor microenvironment driven by tissue heterogeneity or lack of oxygen availability caused more aggressive cells to dominate the tumor and form finger-like protrusions that invade adjacent tissue.
The implication, Dr. Quaranta notes, is that malignant cells are most likely to develop in certain microenvironments and that altering the microenvironment may make tumor cells less invasive.
Importantly, Dr. Cristini explains, published findings from lab experiments using brain tumor cell lines have offered some validation of his team's in silico results. He also has received less formal validation from some neuro-oncology researchers.
"These neurosurgeons are telling me that that's what they have seen over and over in their patients," he recalls. "The model predicted those [tumor shapes] and it did not use any of their data."
Now at the University of Texas School of Health Information Sciences at Houston, Dr. Cristini has begun collaborating with researchers at the University of Texas M.D. Anderson Cancer Center. Using one of the most powerful supercomputers in the world at the Texas Advanced Computing Center, they will run intensive simulations to do things like predict tumor responses to various microenvironmental conditions, including those caused by therapies, and use the simulation results to develop and test new treatment strategies.
Generally speaking, the mathematical models that drive these computational simulations use the available data - both previously published and new experimental data - to populate the "parameters" of the activities they are trying to simulate. This includes, for example, measurements over time of gene and protein activity within cells or of important cell behaviors, such as adhesion to other cells.
Although some models are devoted to whole tumor simulations, Dr. Gallahan notes, a number of models are focused strictly on intracellular signaling networks that control all cellular processes.
Dr. Thomas Deisboeck's computational modeling work, which also has involved brain tumors, represents a blended approach, with a particular focus on how the intracellular signaling pathway directed by the epidermal growth factor receptor (EGFR) influences tumor development.
Dr. Deisboeck, the principal investigator of the Center for the Development of a Virtual Tumor - housed at Massachusetts General Hospital, but composed of researchers from around the world - believes computational modeling is beginning to make important inroads.
"We're...generating exciting hypotheses that can be experimentally tackled and getting data back from those experiments that help to fine-tune and improve our models," he says.
For example, Dr. Deisboeck's team recently collaborated with researchers from the Arizona-based Translational Genomics Research Institute to conduct experiments in cell lines that followed from their in silico simulations. Those simulations assessed EGFR's role in whether brain tumor cells proliferate or migrate (it's believed they tend to do only one or the other at a given time), and the impact those cellular "decisions" have on tumor development. The results (soon to be submitted for publication), he says, "validate our in silico predictions quite nicely."
Such experimental validation is critical to expanding the in silico field and, eventually, integrating computational models into traditional wet lab experiments, clinical trials, and even clinical care, says Dr. Gallahan.
"It's still far off, but we can envision a time when we can establish a specific model for a specific cancer, plug in an individual's own parameters, and see…how an individual will respond to treatment before we even try it, based on a sophisticated model of the cancer process," he says. "The key will be achieving that level of sophistication."
- Carmen Phillips |