How Mycobacteria Make Themselves at Home
 |
David Russell, Ph.D.
Credit: Dr. Russell |
For millennia, a struggle has raged between the human immune system and foreign invaders, such as the bacteria responsible for tuberculosis. Understanding the precise nature of this biological parry and thrust is essential to a better understanding of TB itself, says David Russell, Ph.D., of Cornell University.
Unlike bugs that kill almost every person they infect, Mycobacterium tuberculosis (M. tb) infects many individuals yet causes disease in relatively few. After gaining entry into a person's lungs, the bacteria frequently live unobtrusively for years or decades. During this latent infection, the person generally suffers no obvious disease symptoms and cannot pass on the germ to others. However, if the immune system weakens—whether from age, poor nutrition, or other diseases—the bacteria can emerge from their hiding places and cause full-blown TB.
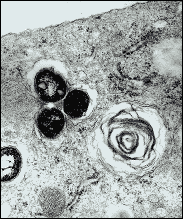 |
Three TB bacteria (dark circular objects) shown inside an animal cell.
Credit: David Russell, Ph.D. |
Much less is known about the habits of latent M. tb than about the actively growing form of the microbe, notes Dr. Russell. Also, the current arsenal of anti-TB drugs is aimed at active M. tb rather than at the more prevalent, persistent form. Now Dr. Russell and his colleagues have gained new insight into the ways M. tb avoids detection and destruction during its quiescent state.
Hiding in Plain Sight
The path to new treatments begins with the interaction between invading bacteria and human macrophages. Macrophages are large immune system cells that rove the body gobbling up potential pathogens. They may destroy the invaders directly or indirectly by recruiting other immune system cells to finish off the pathogen. M. tb avoids elimination by living inside macrophages and using various tricks to prevent the macrophage from doing its job.
In the initial assault on a human lung, TB bacteria multiply rapidly within the macrophages they infect, preventing most macrophages from responding. But some manage to call for help. Soon, a cavalry of other immune system cells swarms in and begins to wall off the infected cells. These walled-off regions, or tubercles, seen on X-rays and in tissue samples from infected lungs, gave the disease its modern name.
Tubercles, also known as granulomas, are advantageous to both the infected person and the bacteria. The granuloma walls off the infection and prevents its spread through the body. But the granuloma itself prevents the immune system from completely eradicating the bacteria. Essentially, M. tb hunkers down in a kind of fortress and begins a long association with its human host in which both respond to the evolving conditions within the granuloma.
A Linchpin Is Found
Dr. Russell joined forces with John McKinney, Ph.D., of The Rockefeller University, and James Sacchettini, Ph.D., of Texas A&M University, to identify an enzyme that M. tb must have to stay alive when it moves into its dormant state.
Once a tubercle forms, the nutrient supply from the human body—which the bacteria need to maintain their waxy coating and stay alive—dwindles. In response, M. tb shifts metabolic gears and begins to use an enzymatic pathway called the glyoxylate shunt. The linchpin of the shunt is an enzyme called isocitrate lyase (ICL). The enzyme is found in bacteria, plants, and some animals, but not in mammals.
|

|
John McKinney, Ph.D.
Credit: Dr. McKinney |
Dr. McKinney created a mutant strain of M. tb that cannot produce ICL. He then infected mice with the mutant bacteria, which functioned perfectly in the initial phase of infection. But when conditions began to approach those of chronic infection, the mutants were unable to adapt. They remained vulnerable to immune system attack and gradually were cleared away.
In cell cultures of macrophages infected with either normal or mutant M. tb, Dr. Russell confirmed that persistent M. tb requires ICL to maintain itself within the macrophage. Without ICL, the bacteria could not keep the immune system at bay. Instead, the macrophages continued to mature after the initial infection and, once activated, wiped out the bacteria. The researchers published their findings in August 2000.
Since their initial studies, the scientists have discovered that not one, but two, genes encode ICL, an indication that the enzyme is even more critical to the TB bug’s survival than they first realized. Notes Dr. McKinney, the glyoxylate shunt is important not only during latent infection, but also throughout the bacterium’s life within the lung. A drug discovery program now underway at the pharmaceutical company GlaxoSmithKline, says Dr. McKinney, has been refined in light of the new understanding about ICL.
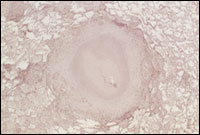 |
Tubercle in human lung
Credit: Gilla Kaplan, Ph.D., Public Health Research Institute |
If scientists could selectively inhibit ICL, they would have a strong weapon specifically aimed at persistent M. tb. There should be no side effects, since humans do not use ICL in any metabolic pathway. Texas A & M's Dr. Sacchettini took a step toward such a specific anti-TB drug when he determined the 3-D structure of ICL using a visualization technique called X-ray crystallography. Dr. Sacchettini's team looked closely at how the shape of ICL changes when it binds to another molecule. This gave them important clues for designing compounds that can prevent ICL from functioning properly.
Another enzyme in the glyoxylate shunt pathway, malate synthase, has captured the attention of Dr. McKinney and his colleagues. It appears that inhibition of the shunt pathway at the malate synthase step might cause a fatal buildup of toxic metabolites in the TB bacteria. Even better, Dr. McKinney adds, the 3-D structure of malate synthase, as determined by Dr. Sacchettini and his co-workers, shows the enzyme’s active site inside a long tunnel of atoms. This, he explains, gives drug designers plenty of “elbow room” to create molecules that might plug the tunnel or otherwise prevent malate synthase from functioning.
References
McKinney, J. D. et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000 Aug 17;406(6797):735-8.
Sharma, V. et al. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8.
Muñoz-Elías, E. J. et al. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005 Jun;11(6):638-44. Epub 2005 May 15.
Rhoades, E. R. et al. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: Characterization of a new model of the granulomatous response to mycobacterial components. Tuberculosis (Edinb). 2005 May;85(3):159-76.
Related Links
Non-government
back to top