
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-69 - Azinphosmethyl
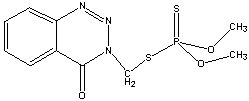
| Chemical Formula: | C10H12N3O3PS2 | - | 3D Structure* |
|---|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | |||
Azinphosmethyl is a broad-spectrum, organophosphorus insecticide that was first produced in 1953 and is used solely for agricultural purposes. In 1974, 3.1 million pounds were estimated to be have been used in the United States on the following crops: alfalfa, cotton, deciduous fruits and nuts, tobacco, vegetables and some miscellaneous items.
A bioassay of technical-grade azinphosmethyl for possible carcinogenicity was conducted by administering the test chemical in feed to Osborne-Mendel rats and B6C3F1 mice.
Groups of 50 rats of each sex were administered azinphosmethyl at one of two doses for 80 weeks, then observed for 34 or 35 weeks. Time-weighted average doses of either 78 or 156 ppm were used for the males. Initial doses of 62.5 or 125 ppm used for the females were maintained throughout the bioassay. Matched controls consisted of groups of 10 untreated rats of each sex; pooled controls consisted of the matched controls combined with 95 male and 95 female untreated rats from similar bioassays of 10 other test chemicals. All surviving rats were killed at 114 or 115 weeks.
Groups of 50 mice of each sex were administered azinphosmethyl at one of two doses for 80 weeks, then observed for 12 or 13 weeks. The doses were either 31.3 or 62.5 ppm for the males and either 62.5 or 125 ppm for the females. Matched controls consisted of groups of 10 untreated mice of each sex; pooled controls consisted of the matched controls combined with 130 male and 120 female untreated mice from similar bioassays of 11 other test chemicals. All surviving mice were killed at 92 or 93 weeks.
High- and low-dose male rats and mice and high-dose female rats and mice had lower mean body weights than corresponding matched controls throughout the bioassay. Typical signs of organophosphate intoxication were observed in a few animals of both species, and included hyperactivity, tremors, and dyspnea. Sufficient numbers of animals were at risk in each species for development of late-appearing tumors.
A great many tumors of the endocrine organs were observed in both dosed male and dosed female rats. Those of the adrenal in dosed males and females, the follicular cells of the thyroid in dosed females, the anterior pituitary in dosed males, and the parathyroid in dosed males occurred at statistically significant incidences when compared with pooled controls, but not with matched controls, and they were not considered to be related to administration of the test compound. The incidences of tumors of the pancreatic islets and of the follicular cells of the thyroid in the male rats suggest, but do not clearly implicate, azinphosmethyl as a carcinogen in these animals.
In mice of each sex there were no increased incidences of tumors that could be related to administration of the test chemical.
It is concluded that under the conditions of this bioassay, neoplasms of the thyroid and pancreatic islets suggest but do not provide sufficient evidence for the carcinogenicity of azinphosmethyl in male Osborne-Mendel rats. Azinphosmethyl was not shown to be carcinogenic in female Osborne-Mendel rats or in B6C3F1 mice of either sex.
Synonym:
0,0-dimethyl-S-[(4-oxo-1,2,3-benzotriazin-3-(4H)-y1)methyl]phosphorodithioate
Trade Name: Guthion®
Levels of Evidence of Carcinogenicity: | ||
| Male Rats: | Equivocal | |
| Female Rats: | Negative | |
| Male Mice: | Negative | |
| Female Mice: | Negative | |
Report Date: 1978
Target Organs from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 15, 2007
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.