
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-67 - Mixture of Aspirin, Phenacetin, and Caffeine
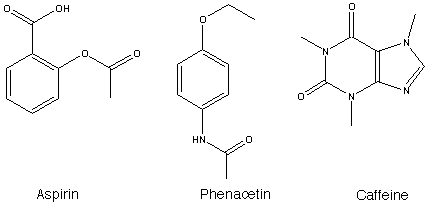
| Chemical Formula: | 3D Structure* | ||
|---|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | |||
| Aspirin | C9H8O4 | ||
| Phenacetin | C10H13NO2 | ||
| Caffeine | C8H10N4O2 | ||
APC, an abbreviation often used for mixtures of aspirin, phenacetin and caffeine, is a nonprescription analgesic preparation sold for relief of headache, muscular aches and pains, arthritis, and other common afflictions. APC is also antipyretic and anti-inflammatory and also acts as a central stimulant.
A bioassay of a mixture of aspirin, phenacetin, and caffeine (APC) for possible carcinogenicity was conducted using Fischer 344 rats and B6C3F1 mice. APC was administered in the feed, at either of two concentrations, to groups of 50 male and 49 or 50 female animals of each species. For each species, 50 animals of each sex were placed on test as controls. The high dose used in the chronic study for the male and female rats and mice was 1.4 percent. The low dose administered to the male and female rats and mice was 0.7 percent. After a 78-week period of compound administration, observation of the rats continued for up to an additional 35 weeks and observation of the mice continued for an additional 16 weeks.
No significant association was established between administration of APC and mortality in rats or female mice; however, there was a significant positive association between treatment and mortality in male mice. For both species the survival in all groups was adequate for statistical analysis of tumor incidence.
In rats, a variety of endocrine tumors were observed with greater frequency in the male rats treated with APC than in the male control rats. These same tumors were not observed with similar frequencies in the female rats or in the mice of either sex. The endocrine tumors observed most frequently in the treated male rats were adenomas and carcinomas of the pituitary gland. The incidences of these tumors proved to be statistically inconclusive.
In rats, a transitional-cell carcinoma of the bladder was observed in one low dose and one high dose females. A tubular-cell adenocarcinoma of the kidney was seen in one low dose female and one low dose male. A fifth neoplasm, a transitional-cell papilloma of the kidney, was seen in one high dose female. The occurrence of these urinary tumors, although considered important, was not statistically significant.
In mice, there was no statistically significant positive association between APC administration and the incidence of tumors in either sex.
Under the conditions of this bioassay evidence was not sufficient for the carcinogenicity of APC in Fischer 344 rats or in B6C3F1 mice.
Levels of Evidence of Carcinogenicity for Aspirin, Phenacetin, and Caffeine Mixture: | ||
| Male Rats: | Negative | |
| Female Rats: | Equivocal | |
| Male Mice: | Negative | |
| Female Mice: | Negative | |
Synonyms for APC: 2-(acetyloxy)-benzoic acid, mixture with
N-(4-ethoxy-phenzl) acetamide and 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione
Synonym for aspirin: acetophenetidine
Synonym for phenacetin: p-acetophenetidide
Synonym for caffeine: theine, 1,3,7-trimethylxanthine
Report Date: 1978
Target Organs from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 15, 2007
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.