|
|
| Unified Medical Language System | |
An Overview to RxNorm |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
I. Introduction and OverviewWhat is RxNorm?RxNorm, a standardized nomenclature for clinical drugs and drug delivery devices, is produced by the National Library of Medicine (NLM). In this context, a clinical drug is a pharmaceutical product given to (or taken by) a patient with a therapeutic or diagnostic intent. A drug delivery device is a pack that contains multiple clinical drugs or clinical drugs designed to be administered in a specified sequence. In RxNorm, the name of a clinical drug combines its ingredients, strengths, and/or form. While ingredient and strength have straightforward meanings, clarification of what is meant by form may be needed. In RxNorm, the form is the physical form in which the drug is administered or is specified to be administered in a prescription or order. The RxNorm clinical drug name does not refer to the size of the package, the form in which the product was manufactured, its form when it arrived at the dispensary or the intended route. RxNorm’s standard names for clinical drugs and drug delivery devices are connected to the varying names of drugs present in many different controlled vocabularies within the Unified Medical Language System (UMLS) Metathesaurus, including those in commercially available drug information sources. These connections are intended to facilitate interoperability among the computerized systems that record or process data dealing with clinical drugs. Purpose of RxNormBecause every drug information system that is commercially available today follows somewhat different naming conventions, a standardized nomenclature is needed for the smooth exchange of information, not only between organizations, but even within the same organization. For example, a hospital may use one system for ordering and another for inventory management. Still another system might be used to record dose adjustments or to check drug interactions. Several cooperating hospitals might have different systems, and find their data incomparable. A standardized nomenclature that relates itself to terms from other sources can serve as a means for determining when names from different source vocabularies are synonymous (at an appropriate level of abstraction). The goal of RxNorm is to allow various systems using different drug nomenclatures to share data efficiently at the appropriate level of abstraction. Objective of this DocumentThis document is intended to give new users a general idea of the purpose and structure of RxNorm. The RxNorm Release Documentation (http://www.nlm.nih.gov/research/umls/rxnorm/docs/index.html) contains a more detailed discussion of the technical aspects of RxNorm. A Simple Idea Implemented RigorouslyRxNorm is organized around normalized names for clinical drugs and drug delivery devices. These names contain information on ingredients, strengths, and dose forms. In the case of the drug delivery devices, the quantity is also listed. For example: For generic drug name- Acetaminophen 500 MG Oral Tablet For a branded drug name- Acetaminophen 500 MG Oral Tablet [Tylenol] For a generic drug pack- {5 (Aspirin 325 MG Oral Tablet) / 5 (Pravastatin 20 MG Oral Tablet) } Pack For a branded drug pack- {30 (Aspirin 325 MG Oral Tablet) / 30 (Pravastatin 20 MG Oral Tablet [Pravachol]) } Pack [Pravigard 325/20] Within RxNorm, generic and branded normalized forms are related to each other and to the names of their individual components by a well-defined set of named relationships. Thus, Acetaminophen 500 MG Oral Tablet is related to Acetaminophen 500 MG Oral Tablet [Tylenol], and both have relationships to Acetaminophen, Acetaminophen 500 MG, and Oral Tablet. Within the UMLS Metathesaurus, Acetaminophen 500 MG Oral Tablet and Acetaminophen 500 MG Oral Tablet [Tylenol] will each be linked to different names that are used for these entities in other vocabularies. The Scope of RxNormRxNorm contains the names of prescription and many nonprescription formulations that exist in the United States, including the devices that administer the medications. RxNorm is intended to cover all prescription medications approved for human use in the United States. Prescription medications from other countries may be included as opportunities allow, a principal consideration being that there be an authoritative source of information about these drugs. Over-the-counter (OTC) medications will be added and covered, as well, when reliable information about the medications can be found. Medications, whether prescription or OTC, with more than four ingredients are not fully represented at the present time. Additions to the vocabulary will be made as new products are put on the market. Radiopharmaceuticals, because of the decay in strength over time and the requirement that they be ordered and prepared especially for a given time of administration, are listed only as ingredients. Contrast media such as barium compounds are also not represented. II. RxNorm in Detail: Structure and OperationHow RxNorm is StructuredAn RxNorm clinical drug name reflects the active ingredients, strengths, and dose form comprising that drug. When any of these elements vary, a new RxNorm drug name is created as a separate concept (explained below). Thus, an RxNorm name should exist for every strength and dose of every available combination of clinically significant ingredients. Nonnumeric named elements of the RxNorm clinical drug name are also individual RxNorm terms related by formal criteria to the clinical drug name. Connections, in the form of predefined relationships, exist among the components of RxNorm and, additionally, between RxNorm data and data derived from other vocabularies also contained in the UMLS Metathesaurus. RxNorm data is distributed in Metathesaurus Relational (MR) or Rich Release Format (RRF) tables. The tables that will be of particular relevance in the following discussion are the following:
For detailed information about the fields, their formatting, and possible values or to learn about other UMLS tables, see the links in the following section. Details of UMLS and RxNorm Structure
The Elements of a Normalized FormRxNorm follows a standard format in the naming of clinical drugs. Drugs named in disparate ways in various other vocabularies are linked to a normalized name prepared according to RxNorm’s naming conventions. The normalized form of the name of a clinical drug may be thought of as being composed of a number of elements, each a concept in its own right. Each element of the normalized form can be identified by the value of the TTY [Term Type] field of RXNCONSO. The possible values are as follows:
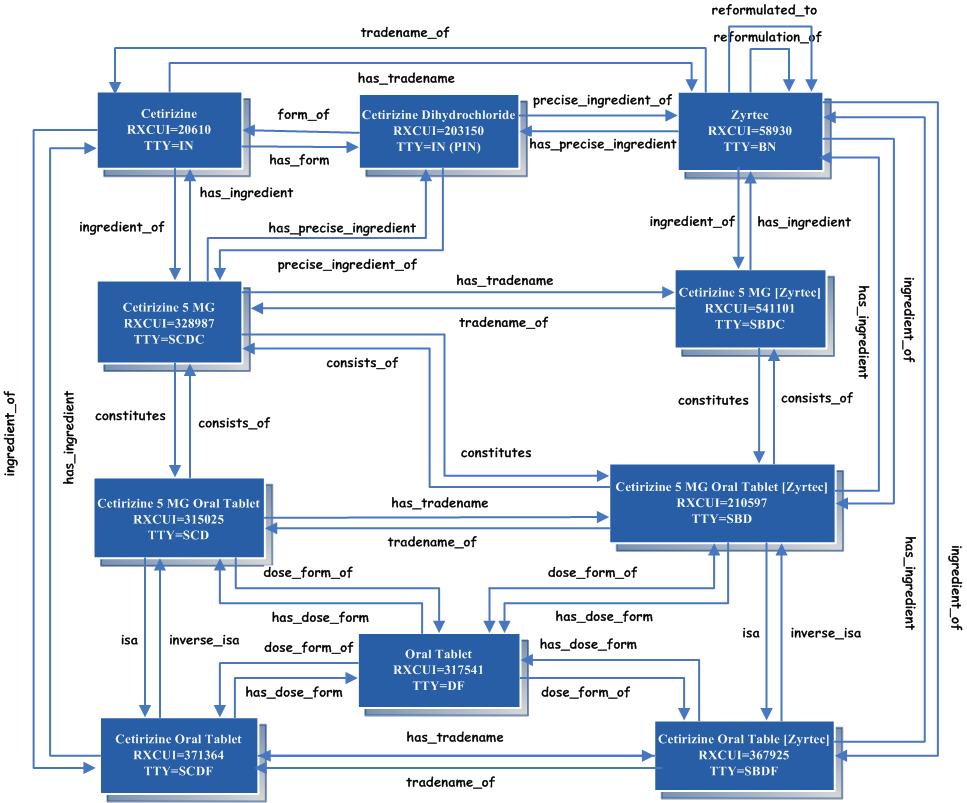
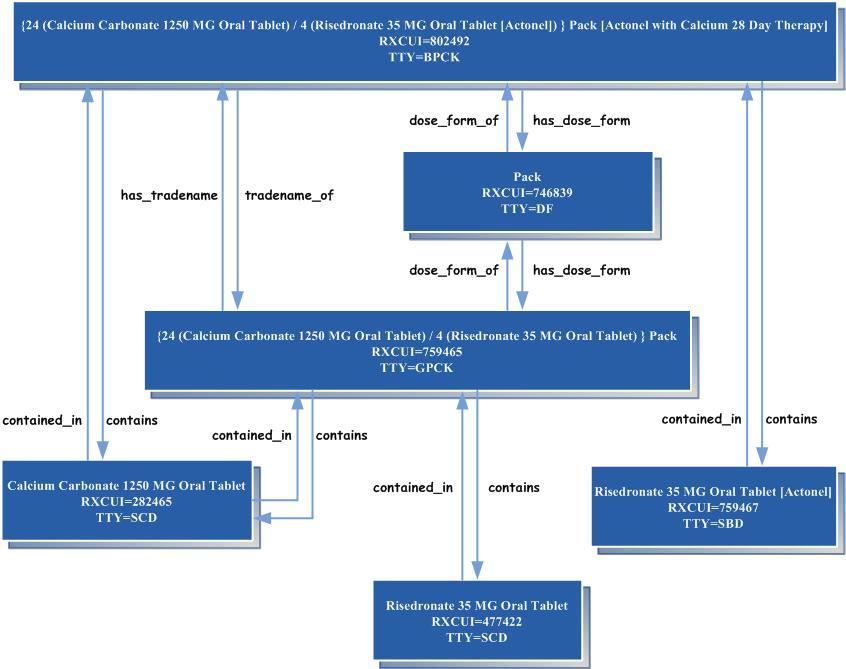
A term type not listed here is OCD (obsolete clinical drug). It is discussed below in the section on Obsolete Records. One final element found in some normal forms is the Quantity Factor. The Quantity Factor is not a separate term type. (See description in technical documentation) A Concept Orientation: RxNorm’s Links to Other VocabulariesLike the UMLS Metathesaurus as a whole, RxNorm is organized by concept. A concept is a collection of names identical in meaning at a specified level of abstraction. It serves as a means whereby strings of characters from disparate sources may be taken to name things that are the same. For example, Accuneb, 0.042% inhalation solution and Albuterol 0.417 MG/ML Inhalant Solution [Accuneb] names the same concept. In RxNorm, where a normalized form exists, it is designated as the preferred form of the name (by means of its association with the TS [Term Status] field in RXNCONSO). The concept is assigned an RxNorm concept unique identifier (RXCUI) of 575803. This RXCUI always designates the same concept, no matter the form of the name and no matter in what table it is found. Drugs whose names map to the same RXCUI are taken to be the same drug—identical as to ingredients, strengths, and dose forms. Conversely, drugs that differ in any of these particulars are conceptually distinct and will have different RXCUIs. Acetaminophen 500 MG Oral Tablet and Acetaminophen 500 MG Oral Tablet [Tylenol], on the other hand, name two different concepts, with RXCUIs 198440 and 209459, respectively. The first of these bears the relationship “has_tradename” to the second and the second bears the reciprocal relationship “tradename_of” to the first. Similarly, two drugs, identical in their generic components, may still refer to different concepts if they differ in brand name. For instance, Fluoxetine 20 MG Oral Capsule [Prozac] andFluoxetine 20 MG Oral Capsule [Sarafem]. RxNorm RelationshipsRelationships between concepts in RxNorm are reciprocal. Each direction of the relationship is represented as a separate row in RXNREL. A clinical drug consists of components, and the components constitute the clinical drug. That is to say, a concept with a TTY field value of SCD bears the relationship “consists_of” to certain other concepts with a TTY value of SCDC, and each of these, in turn, bears the relationship “constitutes” to the first concept. This is shown graphically in the two figures below. For an interactive version of the diagram below, go to RxNorm Concepts and Relationships Diagrams: RXCUI is the concept unique identifier. TTY is the term type. See the section of this document entitled “The Elements of a Normalized Form” above for an explanation of the TTY values.
Relationships at the level of Clinical Drug through the components of each drug to the ingredients
Relationships at the level of Drug Delivery Device to Clinical Drug RxNorm contains the following relationships:
Rules and Conventions Used to Generate RxNorm DataNaming Conventions The SCD—the semantic clinical drug, or normalized form of the generic drug name—always contains the ingredient(s), the strength, and the dose form, in that order. The components and forms of an SCD—its SCDCs and SCDFs—contain the ingredient and strength and the ingredient and dose form, respectively. The SBD follows a similar convention, with the addition of the brand name in brackets at the end of the name. The ingredients named in the SCD, SBD, etc., are the active ingredients. Thus, in the example shown in the figure above, Cetirizine is used as the ingredient name. Though Cetirizine and Cetirizine Dihydrochloride are separate concepts, the normalized form of the drug name does not include the precise ingredient name since, in this case, the difference is without clinical significance. Similarly, RxNorm makes no distinction between amoxicillin trihydrate, amoxicillin monosodium salt, or amoxicillin potassium salt, because the differences among them are not clinically significant. When there are significant differences among components, as is the case with Penicillin G, Benzathine and Penicillin G, Procaine, the entire compound name (the PIN) is always included as the ingredient. Branded DrugsDistinct concepts are created in RxNorm for brands whose formulations (i.e., whose aggregates of ingredients) are distinct. For example, Bactrim and Bactrim DS both contain sulfamethoxazole and trimethoprim (and in the same proportions relative to one another), the DS indicating only that one product is twice as strong as the other. Records for both products link to the same BN. However, in the case of Claritin (loratadine) and Claritin D (loratadine with pseudoephedrine), the “D” indicates an additional ingredient. RxNorm, therefore, contains distinct BNs for both Claritin and Claritin D. If a drug contains one or more components and the proportions are different, this is also reflected in the BN. For example, Advair contains fluticasone and salmeterol. While the amount of salmeterol is constant, the dose of fluticasone can vary. So we end up with several BNs for Advair, including Advair Diskus 500/50 and Advair Diskus 100/50. StrengthsThe strengths are based on the active ingredient. In cases where there is more than one active ingredient, there will be a strength associated with each ingredient, as in the SCD below: Ascorbic Acid 100 MG / Calcium Carbonate 625 MG / Ferrous Fumarate 122 MG / Folic Acid 1 MG Oral Tablet In this example, the SCD bears the relationship “consists_of” to each of the several ingredient-strength pairs (essentially SCDCs) separated by slashes. Strengths are expressed to three significant digits. Thus, nearly equal strengths, which may be expressed differently in different drug vocabularies, are treated as being equivalent. That is, when drug names derived from different source vocabularies would be taken to express the same concept (i.e., to name the same substance), except for a discrepancy in the strengths, and if the strengths given, upon conversion to common units, are identical to three significant digits, RxNorm treats the names as equivalent and assigns the same RXCUI to each string. In most cases, the active ingredient will be the IN. Some drugs, however, will contain a mixture of salts, each of which has a significant and different clinical action. For example, Adderall contains two ingredients, amphetamine and dextroamphetamine. There are six variants of these two ingredients. Four of these variants are clinically active. The RxNorm SCD names each of these salts, with its individual strength, separately and in alphabetical order. Amphetamine Aspartate 1.25 MG / Amphetamine Sulfate 1.25 MG / Dextroamphetamine Saccharate 1.25 MG / Dextroamphetamine Sulfate 1.25 MG Extended Release Capsule In the case of small inorganic molecules, the strength will be expressed in terms of the salt given. For example, an oral tablet that contains 1250 MG of Calcium Carbonate and 500 MG of Calcium will appear as: Calcium Carbonate 1250 MG Oral TabletAs time permits, there will be a SY added to the SCDs that shows both strengths: Calcium Carbonate 1250 MG (Calcium 500 MG) Oral TabletUnits of Measurement In RxNorm only a few units are used, in order to standardize the expressions of strength. Where strengths are expressed as ratios, the ratio is given with a denominator value of 1 of the appropriate units. Thus, 100 mg in 5 ml would be expressed as 20 mg/ml. The following units of measurement are used in RxNorm:
The following unit appears only in ratios:
The following ratios of units have been used in RxNorm:
In making the RxNorm forms, other expressions of units are converted into the RxNorm standards. The rules followed are:
If a variable amount of diluent can be used, the minimum amount is used in RxNorm to calculate the concentration that determines the strength. For example, in the case of a drug that can be dissolved in 3 to 5 ml of diluent, RxNorm would use 3 ml. For drugs with multiple dilution steps, only the initial dilution is used to calculate the strength. For example, a vial containing 50 mg of a drug to be dissolved in 2 ml of water, then added to an IV solution, is expressed as having strength of 25 mg/ml. Reformulated DrugsReformulated drugs are drugs whose ingredients have been changed by the manufacturing company but continue to be identified by the original brand name. A new BN is created in RxNorm to designate the reformulation and provide the date of the change. Sample SBDs are shown below: Dihydroxyaluminum Sodium Carbonate 334 MG [Rolaids] became Because the names and strengths of each component are listed, normal forms may sometimes grow to inordinate lengths. This will be true of multivitamins or ionic solutions such as Lactated Ringer's Irrigation Solution. In such cases, synonyms (TTY=“SY”) will be created in RxNorm as more manageable forms of the name. Strength Expressed as Precise IngredientIf RxNorm receives a string from one of its source vocabularies with the strength expressed in terms of the precise ingredient, this will be noted as an attribute in RXNSAT. See example below: Amiodarone hydrochloride 200 MG Oral Tablet National Drug Code (NDC)Drug products are identified using a unique, three-segment number, called the National Drug Code (NDC). This is used as a universal product identifier for human drugs. There is not a 1 to 1 relationship between the NDC codes and RxNorm forms. One RxNorm form may have many different NDC codes. The NDC is specific down to package size.Conflicts RxNorm obtains NDCs from the Veterans Health Administration (VHA), the Food and Drug Administration, the Centers for Medicare and Medicaid Services (CMS), and the Multum Lexicon. Occasionally (in less than one percent of all cases), conflicts arise among these sources. This may be because of a distinction not made between a branded drug and its generic equivalent or because of a difference in units of measurement (e.g., one source expresses a measurement in terms of weight, another in terms of ionic equivalents) or because of simple data entry error. Instances such as these furnish RxNorm with an important quality assurance opportunity. NDCs are listed as attributes asserted by the source vocabulary. Additionally, RxNorm will assert, as an RxNorm attribute, what is believed to be the correct association with NDC codes. When conflicts arise between sources, other means of obtaining information about the drug may be used in order to determine the correct NDC. In some cases these conflicts arise due to different naming philosophies. While RxNorm considers 21 and 28 day packs of oral contraceptives to be different concepts, some sources group these together and assign multiple NDCs to the same string. RxNorm is creating duplicates of such source asserted atoms in order to properly associate NDC codes with RxNorm forms. When an atom is duplicated, the original source atom is considered to be a 'Base' atom and it is not assigned an RxNorm form. The duplicate atom's string is created by appending '_#N' to the Base atom string where 'N' is a number from 1 to N number of duplicates for this base atom. The duplicate atoms will carry the same source asserted relationships and attributes as the Base atom except for the NDC code attributes. The NDC code attributes of the base atom will be assigned to the duplicate atoms that represent the different meanings of the base atom. RxNorm forms will be created to reflect the true meaning of the NDC code(s) assigned to each duplicate. An NDC code from a base atom can only be assigned to one duplicate atom at a time, but a duplicate atom can carry more than one similar meaning NDC codes from the base atom. The duplicate atom's term type will be created by prepending 'MTH_RXN_' to the base atom's term type. Example of base atoms and duplicate atoms with normal forms: Source Atom: Ortho-Novum 1/35, 35 mcg-1 mg oral tablet_#1Normal Form: {21 (Ethinyl Estradiol 0.035 MG / Norethindrone 1 MG Oral Tablet) / 7 (Inert Ingredients 1 MG Oral Tablet) } Pack [Ortho-Novum 1/35 28 Day] Source Atom: Ortho-Novum 1/35, 35 mcg-1 mg oral tablet_#2 Normal Form: {21 (Ethinyl Estradiol 0.035 MG / Norethindrone 1 MG Oral Tablet) } Pack [Ortho-Novum 1/35 21 Day] Base Atom: Ortho-Novum 1/35, 35 mcg-1 mg oral tablet Codes and CUIsThe values of the Code field (in RXNCONSO, for example) are taken from various source vocabularies and are used, in those sources, to identify particular items in the vocabularies. At one time, RxNorm concepts had a code (used for internal processing) that was different from the RXCUI. Now, all concepts with a Source Abbreviation (SAB) field equal to “RxNorm” have RXCUIs identical to their codes. The following list indicates the fields in the source vocabularies from which the Code is drawn.
For two other sources, the field from which the Code is taken is determined by the term type used in the source record.
The last two MMSL term types are loaded into RxNorm as “NoCode”. NLM is now associating U.S. Food and Drug Administration (FDA) generated unique ingredient identifiers (UNIIs) to RxNorm (SAB=RXNORM) atoms of term type IN. The association is made by an exact string match to the RxNorm ingredient string (case insensitive) from the official FDA substance list. These UNII codes are found in RXNSAT.RRF as values of the attribute ATN='UNII_CODE'. The UNII is a non-proprietary, free, unique, unambiguous, non semantic, alphanumeric identifier based on a substance’s molecular structure and/or descriptive information. For more information on the FDA UNII codes, please refer to this FDA web page. CardinalityWhen a BN has more than one IN, this is noted as an attribute of the BN, with the value “multi”, in table RXNSAT. To find those ingredients, the user should follow the relationship attribute RELA in the RXNREL table (see Appendix 1, showing the values that RELA can take in relation to the term type BN. UpdatesThe full set of files is included in the UMLS Metathesaurus. But while the Metathesaurus, as a whole, is two to three times a year, RxNorm is available as a full update on a monthly basis. In addition, a weekly update containing data from Daily Med and newly approved drugs is available weekly. Between releases of the UMLS, RxNorm update files will be made available through the UMLS Knowledge Source Server. These files will be consistent with the latest extant release of the UMLS, but will contain additional naming information available since that release. It is important to note that the RxNorm files available include names and data from other sources, even though those names are not part of RxNorm. The availability of those names in the RxNorm subset should not be considered as an indication that these can be used without proper consideration of their UMLS licensing restrictions. The restrictions still apply. However, it seems advisable to make those names and relationships available in the updates, in order to support the maintenance of a current drug vocabulary. Because RxNorm is updated so much more frequently than the rest of the UMLS, RxNorm maintains separate concept unique identifiers. At the time of a resynchronized release, UMLS CUIs and RxNorm RXCUIs should have a one-to-one relationship. Tracking changes between versions of the Metathesaurus can be accomplished through the MRCUI file and other Metathesaurus change files. Obsolete RecordsObsolete records are marked in three ways, depending on the source of the record and its relationships with other records. (1) When one of RxNorm’s source vocabularies drops a clinical drug name—that is, when the name had been used in a previous version of that source’s vocabulary but is not found in the most recent version—the old clinical drug records are given the term type OCD (for obsolete clinical drug) in RXNSTY. The record is updated with RxNorm as the source (SAB field), but retains the original SAB, VSAB, TTY, and Code as attributes in RXNSAT. Any existing relationships to RxNorm records will be maintained. (2) When a clinical drug disappears from the U.S. market, the RxNorm SCD should correspond only to OCDs; i.e., it will reside in the same concept exclusively with other records whose TTY fields contain the value OCD. At that time, it is flagged with an “O” (for Obsolete) in the Suppress field in RXNCONSO. (3) If, during a resynchronization with the UMLS (see Updates, above), it is found that there is more than one RxNorm record in the same concept, then one of the records is marked as the preferred one and the others are archived. The archive file is called RXNATOMARCHIVE. Downloading RxNormRxNorm files are available through the NLM download server: http://www.nlm.nih.gov/research/umls/licensedcontent/rxnormfiles.htmland may be downloaded as zipped text files. Because of the difference in update frequency between RxNorm and the rest of the UMLS, the zip files will be the more current. III. RxNorm in UseThe following sample questions and algorithmic solutions illustrate the retrieval of RxNorm information using UMLS data tables. In what follows, field labels are given in capital letters on the left side of the equals sign; field values are given within quotation marks; and field descriptive names, when provided for clarity, are given in square brackets. Taking amoxicillin as exemplar throughout, we can first find all RxNorm concepts related to amoxicillin. In general, other algorithms than the ones shown here may also be available to achieve the same results. 1. In RXNCONSOFind any record with STR[String]=“amoxicillin”. Retrieve RXCUI [Concept Unique Identifier]. NOTE: All strings containing only the word “amoxicillin” will map to the same concept, regardless of capitalization or source vocabulary. That concept, expressed by the RXCUI, will have TTY[Term Type]=“IN” when SAB[Source Abbreviation]=“RXNORM”. 2. In RXNREL Using the RXCUI retrieved from step 1 as the value of RXCUI1, find any record(s) with RELA[Relationship Attribute]=“form_of”. NOTE: RELA is the relationship of RXCUI2 to RXCUI1. Retrieve RXCUI2. NOTE: This step is not strictly necessary in the case of amoxicillin. Its purpose is to retrieve any variants of the active ingredient that might affect the form of the SCD, SCDC, SCDF, SBD, SBDC, or SBDF; i.e., any variants that might be clinically significant. (See discussion above under Rules and Conventions: Naming Conventions.) 3. Let {Ω} stand for the set containing all the RXCUIs retrieved in steps 1 and 2. Q. What trade names is amoxicillin sold under? In RXNRELTaking RXCUI1={Ω}, retrieve the RXCUI2 value for all records with RELA=“tradename_of”. In RXNCONSO Using the retrieved value as the RXCUI in RXNCONSO, find all records with SAB=“RXNORM”. Retrieve STR. NOTE: This retrieves the normalized form of all amoxicillin concepts with TTY= “BN”. Q. What strengths of amoxicillin are available? In RXNRELTaking RXCUI1={Ω}, retrieve the RXCUI2 value for all records with RELA=“has_ingredient”. In RXNCONSO Using the retrieved value as the RXCUI in RXNCONSO, find the records with TTY=“SCDC” for which SAB=“RXNORM”. Retrieve STR. Q. What dose forms of amoxicillin are available? In RXNRELTaking RXCUI1={Ω}, retrieve the RXCUI2 value for all records with RELA=“has_ingredient”. In RXNCONSO Using the retrieved value as the RXCUI in RXNCONSO, find the records with TTY=“SCDF” for which SAB=“RXNORM”. Retrieve STR. Q. What is the National Drug Data File (NDDF) code for amoxicillin? In RXNCONSOUsing the RXCUI retrieved from step 1 as the value of RXCUI1 and SAB=“NDDF,” find the value of the field CODE. Q. Given the NDDF code, how can I find the code for SNOMED, Version 3.5? In RXNCONSOSearch for a record having SAB=“NDDF” and the given value for the CODE field. Retrieve RXCUI. Find a record having that RXCUI and SAB=“SNMI” (abbreviation for SNOMED International from UMLS Knowledge Sources, appendix 2. See the section on Details of UMLS and RxNorm Structures above). Retrieve CODE. Use in Electronic Prescribing and Computerized Order EntryThe representation of the relationships between names of drugs from various drug information sources, their ingredients, strengths, and dose forms should provide a necessary structure for support of electronic prescription writing and physician order entry. However, in some instances, this set of relationships may not be sufficient to convey all of the desired attributes of an order. Some inactive ingredients may still be of interest; e.g., lactose, dextrose, alcohol, flavorings, or dyes. Where packaging of products may be of particular interest (e.g., dose packs), the link between the drug delivery device and the RxNorm form will present the necessary information. In other cases (e.g., vial size or the making up of intravenous solutions), the person preparing the solution will be obligated to review the material at hand, and choose appropriately. Radiopharmaceuticals create a special case. Since they must be specially ordered to have the appropriate radioactivity at a given time, they will not be represented except as a range of approximate activities. |
Last reviewed: 20 April 2009
Last updated: 20 April 2009
First published: 05 May 2005
Metadata| Permanence level: Permanent: Dynamic Content