 |
Research Advances
Volume 11, Number 2
March/April 1996 |
NIDA Research Expands Horizon for Analgesia Alternatives
By John A. Bowersox, NIDA NOTES Contributing Writer
NIDA's commitment to basic neuroscience research continues to result in important advances in the understanding of pain and how it might be controlled more effectively. NIDA-supported studies of the pain-relieving, or analgesic, properties of addictive drugs and how they work have broadened the horizon for analgesia research, as scientists discover that pain relief can be effected through a variety of processes.
"There are multiple pain-relieving mechanisms and many pieces to the puzzle of how they work," says Dr. Lindsay Hough, a NIDA-funded researcher at the Albany Medical College in New York. By putting these pieces together, NIDA-supported researchers hope to develop new pain-relief strategies and improve existing analgesia medications.
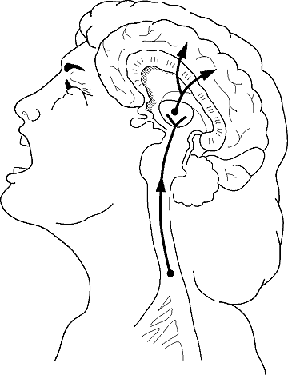 |
|
Opiate drugs produce their analgesic effects by preventing
pain signals from being transferred from the spinal cord to
the brain. One group of researchers is exploring ways to
improve the spinal administration of opiate analgesics tha will
exploit their pain-relieving properties while bypassing their
harmful or unpleasant side effects.
|
A main focus of NIDA-supported analgesia research is to develop medications that relieve pain without producing unwanted side effects. Opiate analgesics such as morphine are among the most effective medications currently available for treating long-term pain. Nonetheless, because many opiates have addictive side effects, physicians often under-prescribe them because they or their patients fear that the use of these medications could lead to opiate addiction. These perceptions linger even though studies have found that the fear of becoming addicted to opiates used clinically to treat pain is unfounded. (See "Pain Relief vs. Physical Dependency and Addiction: A Doctor's Dilemma," NIDA NOTES, July/August 1993, p. 13.)
Still, these fears and debilitating side effects, such as nausea, sedation, confusion, and constipation, that opiate medications can produce limit their effectiveness and contribute to the need for alternative analgesics.
NIDA-supported researchers are addressing this need through a number of experimental approaches. These include:
- developing opioid compounds, synthetic derivatives of opiates, that promote pain relief without producing the euphoria, or "high," that can lead to addiction;
- developing "promoter compounds" that enhance the pain-relieving effects of opioids so that smaller doses can be used; and
- developing nonopioid analgesics that function through a different pain-relief process and presumably will not produce the negative side effects of opioids.
Research conducted by Dr. Hough and his colleagues holds promise for the development of clinically useful nonopioid analgesics. For years, says Dr. Hough, scientists have known that the brain activates a number of pain-relieving systems in response to stress. Several of these systems appear to require histamine, a natural substance released by the body during stress. Histamine produces a variety of effects associated with the stress response, including the stimulation of gastric secretion, the constriction of muscles of the respiratory system, and the dilation of blood vessels.
Dr. Hough added to the number of known effects of histamine by showing that, in rats, morphine does not produce optimal analgesia unless histamine is released from a brainstem structure called the dorsal raphe nucleus or its surrounding tissue, known as the periaqueductal grey. He also found that histamine itself can produce analgesia when injected into this same part of the brain. Histamine-induced analgesia can be inhibited by simultaneously injecting morphine antagonists, compounds that interfere with the interactions between morphine and brain cells, into the same brain region. These findings, he says, suggest that histamine works with morphine or other opioid compounds to relieve pain through a shared brain pathway.
In recent studies, Dr. Hough and his colleagues showed that certain compounds derived from histamine can induce powerful analgesia in rats. Most importantly, says Dr. Hough, the histamine derivatives, unlike histamine, are not blocked by opiate antagonists. This finding implies the existence of a pain relief pathway in the brain that functions independently of the pathway used by morphine and other opiates.
"We think the histaminergic system is central to analgesia," says Dr. Hough, referring to the nerve cells and brain regions that use histamine as their neurotransmitter, or chemical messenger. Although still at an early stage, Dr. Hough's research into histamine-derived analgesics could lead to a new class of nonopioid pain relief medications.
While NIDA-supported researchers such as Dr. Hough are trying to develop novel pain-relief strategies that are not mediated by opioid compounds, others are trying to improve existing opioid medications by finding ways to enhance their analgesic properties.
Studies have found that the
fear of becoming addicted to
opiates used clinically to
treat pain is unfounded
Researchers have long known that amphetamines and other central nervous system stimulants increase the analgesia induced by opioid medications. Anecdotal evidence suggests that medications that activate brain regions that use the neurotransmitters serotonin and norepinephrine can produce similar effects. NIDA-funded researchers hope to exploit these effects through the development of a pain-relief strategy known as enhancement or promoter therapy.
The principal aim of enhancement therapy research is to identify nonanalgesic drugs that can selectively enhance the pain-relieving effects of morphine and other opiates, says NIDA researcher Dr. Danny Shen. This approach ultimately could allow physicians to prescribe smaller doses of opioids while achieving the same or greater degrees of pain relief. Smaller doses of opioids also could make the detrimental side effects of these medications less of a consideration in treatment decisions.
Dr. Shen and Dr. Barbara Coda, his colleague at the Fred Hutchinson Cancer Research Center in Seattle, are investigating whether compounds that either promote or prolong the activity of serotonin, a neurotransmitter that some neurons use to communicate with each other, can enhance analgesia.
In a NIDA-supported study, these investigators confirmed the long-held belief that activation of the brain's serotonergic pathways can enhance opiate-induced analgesia in people. In this study, subjects who were given fenfluramine, an appetite suppressant that promotes the release of serotonin from neurons, required 25 to 50 percent less morphine to relieve their pain than did those who received a placebo.
Dr. Shen is continuing this research to test a class of antidepressant medications known as selective serotonin re-uptake inhibitors (SSRIs). As their name suggests, SSRIs inhibit serotonin-releasing neurons from taking the neurotransmitter back up after it has been released. Thus, serotonin remains in the spaces between neurons, free to repeatedly activate those that have serotonin receptors.
A main focus of NIDA-supported
analgesia research is to develop
medications that relieve pain
without producing unwanted
side effects.
A consortium of NIDA-supported investigators that includes Dr. Shen, Dr. Christopher Bernards of the University of Washington, and Dr. Tony L. Yaksh of the University of California at San Diego is trying to determine the best way to deliver opiate analgesics directly to the spinal cord. Dr. Yaksh was the first to demonstrate that opiates with an action limited to the spinal cord could produce a powerful analgesia. This work has led to the characterization of other spinal receptor systems that also can produce analgesia.
Spinal drug delivery reduces the discomfort that morphine can cause. However, some morphine is still able to seep into the bloodstream and thus be carried into the brain and other organs, where opiates' side effects originate. Researchers suspect that improving the spinal administration of opioids could present yet another opportunity to exploit the analgesic properties of opiates while bypassing their harmful or unpleasant side effects.
Sources
Coda, B.A.; Hill, H.F.; Schaffer, R.L.; Luger, T.J.; Jacobson, R.C.; and Chapman, C.R. Enhancement of morphine analgesia by fenfluramine in subjects receiving tailored opioid infusions. Pain 52:85-91, 1993.
Thoburn, K.K.; Hough, L.B.; Nalwalk, J.W.; and Mischler, S.A. Histamine-induced modulation of nociceptive responses. Pain 58:29-37, 1994.
Yaksh, T.L., and Rudy, T.A. Analgesia mediated by a direct spinal action of narcotics. Science 192:1357-1358, 1976.
From NIDA NOTES, March/April, 1996
[NIDA Home Page][NIDA NOTES Index][1996 Archive Index Index]
|