 |

Study Suggests New Options for Follicular Lymphoma
Treatment with a radiolabeled monoclonal antibody appears to be highly effective as consolidation after first-line therapy in patients with indolent, advanced-stage follicular lymphoma, according to results of a large phase III clinical trial published October 14 in the Journal of Clinical Oncology (JCO).
The results showed that consolidation therapy - a form of adjuvant therapy after initial or "induction" therapy to induce remission - with a single dose of yttrium-90 (90Y)-ibritumomab tiuxetan (Zevalin) significantly improved progression-free survival in all patient subgroups compared to patients in the control arm, who received induction therapy but not consolidation therapy. 90Y-ibritumomab is a monoclonal antibody with a radioactive isotope attached to it.
The improvement in progression-free survival held up regardless of whether patients had a complete response (CR) or partial response (PR) to induction therapy: 53.9 months vs. 29.5 months for CR and 29.3 months vs. 6.2 months for PR. In addition, the multinational research team, led by investigators at the Universitair Medisch Centrum Utrecht in the Netherlands, reported 77 percent of patients in the treatment arm with a PR to induction therapy "converted" to a CR after receiving 90Y-ibritumomab. Read more 


Gene Signature May Predict Recurrence of Liver Cancer
Researchers have enhanced a genetic technique for studying chemically preserved tissue samples and used it to discover a gene signature that may identify patients with liver cancer who are at risk of a recurrence. Their findings also suggest that some late recurrences (more than 2 years after the initial disease) may not be recurrences at all, but rather new primary tumors that develop in a liver damaged by environmental factors such as infection by a hepatitis virus or cirrhosis.
In the study, published online October 15 in the New England Journal of Medicine (NEJM), Dr. Todd Golub of the Dana-Farber Cancer Institute and his colleagues first overcame a technical challenge. Genome-wide expression profiling studies, which are large-scale surveys of gene activity in cells, have led to new classifications of cancers and diagnostic tools, but they can only be performed on frozen samples. Vast stores of patient samples, including specimens with long-term clinical follow-up, have been preserved with the chemical formalin rather than by freezing, and therefore are unavailable for genomic analyses.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Study Suggests New Options for Follicular Lymphoma
Treatment with a radiolabeled monoclonal antibody appears to be highly effective as consolidation after first-line therapy in patients with indolent, advanced-stage follicular lymphoma, according to results of a large phase III clinical trial published October 14 in the Journal of Clinical Oncology (JCO).
The results showed that consolidation therapy - a form of adjuvant therapy after initial or "induction" therapy to induce remission - with a single dose of yttrium-90 (90Y)-ibritumomab tiuxetan (Zevalin) significantly improved progression-free survival in all patient subgroups compared to patients in the control arm, who received induction therapy but not consolidation therapy. 90Y-ibritumomab is a monoclonal antibody with a radioactive isotope attached to it.
The improvement in progression-free survival held up regardless of whether patients had a complete response (CR) or partial response (PR) to induction therapy: 53.9 months vs. 29.5 months for CR and 29.3 months vs. 6.2 months for PR. In addition, the multinational research team, led by investigators at the Universitair Medisch Centrum Utrecht in the Netherlands, reported 77 percent of patients in the treatment arm with a PR to induction therapy "converted" to a CR after receiving 90Y-ibritumomab.
"This constitutes one of the highest PR-to-CR conversion rates reported in published phase III randomized studies in first-line follicular lymphoma," they wrote. Considering the efficacy and modest toxicity associated with 90Y-ibritumomab in the trial, consolidation therapy with this monoclonal antibody "may be considered as part of the current treatment algorithm of follicular lymphoma," they concluded.
Seattle-based Cell Therapeutics, Inc., which manufactures 90Y-ibritumomab, is now seeking FDA approval to begin marketing the drug for first-line consolidation therapy in patients with follicular lymphoma, a type of non-Hodgkin lymphoma. 90Y-ibritumomab is already approved in the United States to treat follicular lymphoma that has recurred or is unresponsive to available treatment regimens.
The Firstline Indolent Trial included 414 patients from 77 medical centers across Europe. Patients received a variety of induction therapy regimens, which included different chemotherapy agents and, in approximately 15 percent of patients, also included the monoclonal antibody rituximab (Rituxan). After induction therapy, patients were randomized to no consolidation therapy or consolidation with a two-course "priming" dose of rituximab followed by a single dose of 90Y-ibritumomab. Because of the limited follow-up, there are no data from the trial yet on overall survival.
Given the positive results seen even in patients who received less aggressive induction regimens, the authors added, 90Y-ibritumomab "has the potential to maximize tumor response without using aggressive induction regimens and may reduce the need for aggressive chemotherapy."
The limited use of rituximab as part of induction therapy in the trial hinders the interpretation of its results, Dr. Oliver W. Press, chair for lymphoma research at Fred Hutchinson Cancer Research Center, cautioned in an accompanying editorial in JCO. While calling the trial a "landmark study," he noted that, based on results from several phase III clinical trials, combining rituximab "with each cycle of induction chemotherapy has become a worldwide standard."
Even so, says Dr. John Leonard, director of the Center for Lymphoma and Myeloma at Weill Cornell Medical College in New York, its good safety profile and the fact that it is well tolerated by patients makes 90Y-ibritumomab "particularly attractive for [use in] older patients," who may not handle more toxic chemotherapy regimens very well.
Radioimmunotherapy, as the use of radiolabeled monoclonal antibodies is often called, has not been widely adopted, Dr. Leonard notes, in part because it's not as easy for oncologists to use as other available treatments, including rituximab. For one thing, it has to be administered in a facility specially equipped to handle radioactive compounds.
A number of trials, Dr. Leonard continues, are ongoing that should help to clarify the role of radioimmunotherapy in patients with
newly diagnosed, advanced follicular lymphoma.
—Carmen Phillips
|
 |
 |
Gene Signature May Predict Recurrence of Liver Cancer
Researchers have enhanced a genetic technique for studying chemically preserved tissue samples and used it to discover a gene signature that may identify patients with liver cancer who are at risk of a recurrence. Their findings also suggest that some late recurrences (more than 2 years after the initial disease) may not be recurrences at all, but rather new primary tumors that develop in a liver damaged by environmental factors such as infection by a hepatitis virus or cirrhosis.
In the study, published online October 15 in the New England Journal of Medicine (NEJM), Dr. Todd Golub of the Dana-Farber Cancer Institute and his colleagues first overcame a technical challenge. Genome-wide expression profiling studies, which are large-scale surveys of gene activity in cells, have led to new classifications of cancers and diagnostic tools, but they can only be performed on frozen samples. Vast stores of patient samples, including specimens with long-term clinical follow-up, have been preserved with the chemical formalin rather than by freezing, and therefore are unavailable for genomic analyses.
To address this problem, scientists at Illumina, Inc., recently developed a technique for profiling several hundred genes in formalin-fixed samples. In this study, Dr. Golub and his colleagues modified the technique to profile approximately 6,000 genes. They obtained useful information from 90 percent of the 2,000 patient samples they examined, including some collected 24 years ago.
Next, the researchers profiled liver tumors, but failed to detect a survival signature. They then hypothesized that tissues surrounding the liver tumor - rather that the tumor itself - might harbor a genetic signature associated with recurrence. This adjacent tissue is frequently abnormal in patients who have experienced infection or cirrhosis.
Using a set of 106 patients, the team identified a 186-gene signature based on the activity of genes in tissues adjacent to tumors. They validated it using samples from patients in other parts of the world. If confirmed by future studies, the signature could identify at-risk patients who might benefit from intensive treatments and preventive measures.
The ability to survey the entire genome in an unbiased manner was essential, noted Dr. Golub, who also directs the cancer program at the Broad Institute. "We never would have discovered this signature if we had only been profiling several hundred genes," he said. A commerical tool is now available for genome-wide analyses of formalin-fixed samples.
One in Four Teenage Girls Receives HPV Vaccine
One in four teenage girls has begun the process of vaccination against human papillomavirus (HPV) with the three-shot series of Gardasil, according to the 2007 National Immunization Survey-Teen. In March 2007 the Advisory Committee on Immunization Practices (ACIP) recommended that all girls age 11 or 12 be routinely vaccinated with three doses of quadrivalent HPV vaccine. The new survey was the first official government report on compliance.
The vaccination series can be started as young as age 9. The ACIP also advises females aged 13 to 26 to obtain "catch-up" vaccinations,
even though Gardasil is preventive, not therapeutic, and they may have already been exposed to HPV. The quadrivalent vaccine protects against HPV types 6, 11, 16, and 18, which account for up to 70 percent of cervical cancers and 90 percent of genital warts.
The 25.1 percent vaccination rate drawn from the sample group suggests that 2.5 million of the 10 million teenage girls in the United States were vaccinated. However, the survey was completed by telephone interview in 5,474 households where a teen boy or girl aged 13 to 17 lived, and results were confirmed from medical records for only 2,947 of those teens. Furthermore, the vaccination schedule calls for three shots over the course of 6 months; thus those surveyed as having received one dose of the vaccine may not complete the full, three-shot series. Finally, some structural aspects of the survey methodology and health care provider histories could make it difficult to generalize these results either to the entire population or to specific groups, such as Latina teens.
The results were published October 10 in the Morbidity and Mortality Weekly Report, as part of a report on recommended vaccinations for adolescents. It also included compliance reports for several other ACIP-recommended vaccines indicated for adolescents.
Smoking in Middle Age Decreases Quality of Life in Old Age
It is well established that smoking shortens life expectancy, but smoking in midlife also results in a poorer quality of life in old age, according to a study published October 13 in Archives of Internal Medicine.
Researchers in Finland examined the relationship between long-term cigarette smoking and health-related quality of life (HRQoL) among 1,658 men participating in the Helsinki Businessmen Study. All the men were healthy at baseline in 1974 with a mean age of 47.8 years. The health status of 1,131 (87.9 percent) of those men alive in 2000 was re-evaluated using the RAND 36-Item Health Survey, a widely used measure of HRQoL. The researchers found that participants who never smoked lived, on average, 10 years longer than heavy smokers and had the highest (i.e., best) scores on the RAND scales.
"The differences were greatest between never smokers and heavy smokers, ranging from 4 points on the scale of social functioning to 14 points on the physical functioning scale," the scientists reported. The physical component summary score showed a graded deterioration of HRQoL as the number of cigarettes smoked daily increased.
Compared with heavy smokers, never smokers "enjoyed significantly better health status in late life, which was equal to an age difference of 10 years in the general population," the researchers noted. "From a prevention point of view, our findings of the smoking/HRQoL relationship add to the view of the burden of smoking on society. The argument of better quality of life may be especially meaningful for the aging smoker but, as our results show, for the best HRQoL [smoking] should not be started at all."
More Evidence for Role of ALK in Neuroblastoma
In August 2008, researchers identified germline mutations in the anaplastic lymphoma kinase (ALK) gene in the vast majority of families with the inherited form of neuroblastoma. They also found somatic ALK mutations in 12 percent of high-risk, spontaneous (not inherited) tumor samples. An additional four studies published in the October 16 Nature have now confirmed a role of ALK in neuroblastoma.
In the first study, investigators found three different germline mutations in ALK in eight families affected by hereditary neuroblastoma, and somatic ALK mutations in 8.4 percent of samples from spontaneous tumors and 35.7 percent of neuroblastoma cell lines. In the second study, researchers found germline ALK mutations in two out of six families, and discovered that gene number alterations and gene changes in ALK in spontaneous tumors occurred mainly in two regions on the gene.
The third study also found ALK mutations in 6.1 percent of primary tumors and 33 percent of neuroblastoma cell lines. The fourth study identified five different ALK mutations in 8 percent of neuroblastoma samples; three were somatic and two were germ-line mutations.
Importantly, these mutations appear to increase the activity of the ALK protein; the studies showed that the mutant ALK protein was overexpressed and continually activated other proteins in downstream cell-signaling pathways, which can lead to aberrant cell growth and division. Inhibiting the mutant ALK genes, either with RNA interference or with targeted drugs, decreased the proliferation of neuroblastoma cells or caused cell death in all of the studies.
"Neuroblastoma is the most common childhood cancer diagnosed before the age of one, and accounts for some 15 [percent] of all cancer deaths in children," says Dr. Charis Eng from the Cleveland Clinic in an accompanying editorial. Although further studies will be needed to define how ALK might be targeted for treatment or for genetic testing in families affected by inherited neuroblastoma, she explains, "The long-awaited discovery of a major non-syndromic neuroblastoma gene is indeed a welcome advance."
|
 |
 |

Remembering a Leading Advocate for Cancer Research
Last week the country lost a true champion of biomedical research and proponent of quality medical care for every American. Former Congressman Paul G. Rogers, one of the principal leaders in the development and passage of the National Cancer Act of 1971, died on October 13. He was 87.
 I can only imagine that as a young man whose formative experiences included serving in the U.S. armed forces during World War II - for which he received the Bronze Star - Congressman Rogers could not have foreseen that he would become one the most prominent and well-respected voices in support of biomedical research and health care our country has ever known. I can only imagine that as a young man whose formative experiences included serving in the U.S. armed forces during World War II - for which he received the Bronze Star - Congressman Rogers could not have foreseen that he would become one the most prominent and well-respected voices in support of biomedical research and health care our country has ever known.
Even as he began the first term of an illustrious 24-year career as a member of the U.S. House of Representatives, he could not have anticipated that he would be honored by organizations like the American Cancer Society, the National Osteoporosis Foundation, and the Lasker Foundation; or that he would be elected to such prestigious organizations as the Institute of Medicine; or that he would have a plaza on the NIH campus named in his honor.
But if his actions are any indication, he truly lived his public life by his own words, which are now inscribed on a plaque in the Paul G. Rogers Plaza on the NIH campus: "Without research, there is no hope."
Congressman Rogers' accomplishments are both legion and legendary. During his 8-year tenure as chair of the House Subcommittee on Health and the Environment, he ushered through the passage of the Health Manpower Training Act; National Heart, Blood Vessel, Lung, and Blood Act; Safe Drinking Water Act; and Community Health Centers Act, just to name a few. As these laws demonstrate, although Congressman Rogers believed in the power of research, he also knew that maintaining and improving public health extended well beyond the walls of the laboratory.
Congressman Rogers was committed to ensuring that research being done at government and large academic centers was translated and delivered to the communities where most people receive their care. As he recounted in the NCI Cancer Bulletin nearly 2 years ago, among the most important provisions in the National Cancer Act were those that established the Cancer Centers Program. The continued growth of that program is a testament to Congressman Rogers' foresight and wisdom as a policy maker. The launch last year of the NCI Community Cancer Centers Program pilot is, in many ways, an extension of Congressman Rogers' fundamental belief in the importance of ensuring the translation and delivery of cutting-edge cancer care to patients where they live.

Congressman Rogers was prescient about the challenges of making quality medical care, including cancer care, available to every American, regardless of race, culture, or socioeconomic status. In a 1991 interview, he talked about the need to address the growing number of Americans without health insurance or without "adequate access to the [health care] system." He also spoke about the growing burden of the escalating cost of care. Both are issues we are still trying to come to grips with today.
Indeed, in a 1979 interview, the year he left public office, he spoke of what drove his legislative efforts in health care. "I saw the potential for what could be done…and it was just not being aggressively pursued," he said. "We were not looking ahead and planning."
As a legislator, a policy maker, a mentor, and a public health leader, Congressman Rogers' efforts have had a profound impact on biomedical research and medical care in the United States. His tireless efforts, it can be safely said, have helped build infrastructure and establish policies that will ensure continued progress for years to come.
I have lost a friend and mentor. Paul was among the first to welcome me to Washington, D.C., and to my new position at NCI. He was eager to introduce me to his many friends and was always full of encouragement whenever our paths crossed. He never saw me without saying, "We have to get you more money to do your job." He understood the importance of our nation's investment in biomedical research. I will remember his gracious manner and will do my best to follow the high standards he set for all of us.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |

Research Portfolio Offers More Information, New Tools
NCI has launched a revamped version of the NCI-Funded Research Portfolio (NFRP) on Cancer.gov. The revision consolidates information that was previously available across various pages of NCI's Web site, including funding and data on NCI's intramural research program, and makes available information not previously found on Cancer.gov.
Users can search individual disease categories and specific conditions, or use a more overarching approach to search by year of funding or key words. The research project records yielded by the search include a link to the abstract that explains their methodology and goals.
Users can also select how they would like to view the output of their searches: either a detailed "coding" report that shows all of the codes NCI's expert indexers used to categorize each research project or grant, or a "budget" report that details the proportional relevance of that project to a given category and the prorated dollars to a given category. This information can be exported into an Excel spreadsheet.
The new NFRP site can be accessed from various points on Cancer.gov, including the "Research and Funding" tab along the top, or the "Science Serving People" or "Director's Corner" portals. The NFRP will continue to undergo refinements in the coming months. Users are encouraged to submit their comments to NCIfundedportfolio@mail.nih.gov.
|
 |
 |

Lung Cancer and Erlotinib: Which Patients Benefit?
One of the most promising recent developments in cancer medicine has been the dramatic responses of some patients with lung cancer to the drugs gefitinib (Iressa) and erlotinib (Tarceva). Although only about 10 percent of patients with lung cancer respond in this way, a larger group experiences prolonged survival and an improvement in symptoms. Unfortunately, the best way to identify candidates for these drugs, called EGFR tyrosine kinase inhibitors (TKIs), is not yet clear.
 NCI has launched a clinical trial called MARVEL (Marker Validation of Erlotinib in Lung Cancer) that could offer guidance. The study aims to validate a candidate biomarker for response to erlotinib: extra copies of the epidermal growth factor receptor (EGFR) gene in lung tumors. Several recent studies have suggested that patients who harbor this gene "amplification" may benefit from erlotinib. The trial will also assess other candidate prognostic markers such as EGFR gene mutations. NCI has launched a clinical trial called MARVEL (Marker Validation of Erlotinib in Lung Cancer) that could offer guidance. The study aims to validate a candidate biomarker for response to erlotinib: extra copies of the epidermal growth factor receptor (EGFR) gene in lung tumors. Several recent studies have suggested that patients who harbor this gene "amplification" may benefit from erlotinib. The trial will also assess other candidate prognostic markers such as EGFR gene mutations.
"This study represents a unique opportunity for the prospective validation of multiple markers," said co-principal investigator Dr. Fred R. Hirsch of the University of Colorado Health Sciences Center. He and the group at the University of Colorado Cancer Center pioneered the use of fluorescence in situ hybridization (FISH) as a means of detecting EGFR amplifications in lung tumors, and the technology will be evaluated in MARVEL along with the biomarker itself.
Breast cancer tumors are routinely analyzed using FISH to identify women who are candidates for trastuzumab (Herceptin), and the technology could readily be adapted for clinical use in lung cancer.
In the randomized trial, approximately 1,200 patients with advanced non-small-cell lung cancer (NSCLC) who were treated previously will receive erlotinib or pemetrexed, a standard second-line chemotherapy drug for lung cancer. The investigators hypothesize that erlotinib will be superior in patients who are EGFR-positive as detected by FISH, while the chemotherapy will be superior in those who test EGFR-negative.
"This study has two main purposes - to define which population will benefit the most from receiving EGFR-targeted therapies, and to validate some of the tests used to measure the EGFR target," said Dr. Claudio Dansky Ullmann of NCI's Division of Cancer Treatment and Diagnosis, who, along with Dr. Janet Dancey, formerly of NCI and now at the Ontario Institute for Cancer Research, helped develop the trial.
Multiple cancers involve the activation of the EGFR gene, either through mutation or increases in the number of gene copies of EGFR. Though EGFR gene mutations are associated with strong responses to erlotinib, sometimes lasting several years, the mutations are rare in Western populations. By comparison, approximately 50 percent of NSCLCs may harbor increased EGFR gene copy number. This suggests that the change, if validated, could be broadly useful as a biomarker of response, noted Dr. Hirsch.
"Our hope is that the trial will lead to the identification of markers that will ultimately allow the selection of a specific treatment that is likely to be the most effective for individual patients," said Dr. Dancey.
Along with molecular markers, clinical characteristics such as being a female, Asian ethnicity, and smoking fewer than 100 cigarettes in a lifetime are associated with response to EGFR TKIs. Responders also tend to have lung adenocarcinomas.
The trial will also evaluate mutations in the KRAS gene as possible biomarkers. In colorectal cancer, KRAS mutations are associated with resistance to cetuximab (Erbitux), a monoclonal antibody that targets the EGFR protein, but there is insufficient evidence to draw conclusions about these mutations in lung cancer.
"We expect that this study will teach us a lot about how the different EGFR biomarkers correlate to each other," said Dr. Hirsch, "and also about their associations with clinical outcomes."
The multicenter study is NCI's first to determine prospectively whether biomarkers can help guide treatment for lung cancer. Many partners have been involved in the planning and design from the outset, including NCI cooperative groups, the Food and Drug Administration (FDA), and the Centers for Medicare and Medicaid Services.
"The MARVEL trial is unique and an important first because it is an outgrowth of specific NCI initiatives designed to advance lung cancer therapies and received broad input from the FDA, NCI cooperative groups, the biomarker industry, and the pharmaceutical industry," said Dr. Alex A. Adjei, of the Roswell Park Cancer Institute and chair of the study, in a statement.
—Edward R. Winstead
|
 |
 |

Preventing Respiratory Infections in Patients with CLL
Name of the Trial
Phase III Randomized Study of American Ginseng Extract to Prevent Respiratory Infection and Reduce Antibiotic Use in Patients with Chronic Lymphocytic Leukemia (CCCWFU-98308). See the protocol summary at http://www.cancer.gov/clinicaltrials/CCCWFU-98308.
Principal Investigators
Drs. Kevin High, Bayard Powell, David Hurd, Denise Levitan, and Leslie Ellis, Wake Forest University CCOP Research Base
Why This Trial Is Important
Chronic lymphocytic leukemia (CLL) is a type of blood cancer that progresses very slowly over many years. Most patients are started on treatment only when needed. Both CLL and its treatment can impair immune system functioning and markedly increase risk of infection, the most common complication of CLL.
The advanced age of most people with CLL (70 percent over age 65) is also a risk factor for infection. Acute respiratory infection (ARI) is the most common infection in CLL patients. Although there are no published data on the occurrence of ARI in untreated CLL patients, these individuals appear to be more prone to infection than people of similar age with non-cancerous conditions. Recent studies suggest the average number of days with ARI during the winter season is about 8 among cancer-free individuals who are roughly the same age as CLL patients.
Two randomized, controlled trials have shown that an extract of
North American Ginseng, called CVT-E002 (COLD-fX), can significantly reduce the risk of ARI in older adults. Other research suggests that the active ingredient in CVT-E002 enhances the function of certain white blood cells (macrophages and natural killer cells) that are part of the immune system.
These results led to the development of this randomized, double-blind, placebo-controlled study to test the ability of CVT-E002 to reduce ARI and the need for antibiotic treatment during the peak respiratory illness season (January through March) in people with CLL. All patients will be enrolled between November 1 and December 31, 2008. Once patients are identified and informed consent obtained, they will be randomly assigned to receive either CVT-E002 (200 mg orally twice a day) or matching placebo pills. Treatment will continue through April 30, 2009. The researchers want to see if CVT-E002 can reduce the number of days with ARI by 30 percent, as well as antibiotic use among CLL patients over a 3-month period (January 1-March 31).
For More Information
See the lists of entry criteria and trial contact information at http://www.cancer.gov/clinicaltrials/CCCWFU-98308 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |

Delving Deeper into Exercise and Breast Cancer Prevention
For women concerned about breast cancer, looking over the list of known risk factors can be cause for frustration, as few of the stronger risks appear to be modifiable. But this may change as more is learned about the role of exercise in preventing breast cancer.
 A growing body of research indicates that the levels of hormones produced by the body can be modified by physical activity. While a woman cannot change her age at menarche or menopause, and may not have children or breast feed - all factors which affect the levels of ovarian hormones a woman is exposed to over her lifetime - many researchers believe that she can reduce her exposure to these hormones through exercise. A growing body of research indicates that the levels of hormones produced by the body can be modified by physical activity. While a woman cannot change her age at menarche or menopause, and may not have children or breast feed - all factors which affect the levels of ovarian hormones a woman is exposed to over her lifetime - many researchers believe that she can reduce her exposure to these hormones through exercise.
Ovarian hormones including estrogen and progesterone, for example, are necessary for a woman's reproductive and overall health, but they also stimulate breast cells to proliferate, which can increase the accumulation of genetic mutations and potentially lead to tumor formation.
"Our theory is that the accumulative number of ovulatory menstrual cycles a woman experiences is predictive of her breast cancer risk," explains Dr. Leslie Bernstein, professor of cancer etiology at City of Hope Comprehensive Cancer Center.
She and her colleagues have been involved in the California Teachers Study (CTS) since 1995, which enrolled 133,479 current and retired teachers and school administrators in the state of California to prospectively study potential causes of an excess incidence of breast and other cancers observed in this population.
Last year the CTS investigators found that the risk of invasive breast cancer was inversely associated with long-term strenuous physical activity - women who averaged more than 5 hours a week of strenuous physical activity between high school and their current age (or age 54 if 55 or older) had a significantly reduced risk compared to women who averaged less than half an hour of strenuous physical activity a week during the same period of life.
Unexpectedly, neither strenuous nor moderate long-term physical activity was associated with reduced risk of estrogen receptor (ER)-positive breast cancer, but women who participated in long-term strenuous physical activity had a 55 percent reduction in risk of ER-negative breast cancer, and women who participated in long-term moderate physical activity had a 47 percent reduction in risk.
While drugs such as tamoxifen and raloxifene can help prevent the formation of ER-positive breast cancer, no such agents currently exist that can help prevent ER-negative cancer, making interventions to reduce the risk of ER-negative cancer "critically important," says Dr. Bernstein.
Investigators from NCI's Division of Cancer Epidemiology and Genetics (DCEG) recently obtained similar results from their National Institutes of Health-American Association of Retired Persons (AARP) Diet and Health Study, another prospective cohort study begun in 1995. They examined current physical activity among women aged 50 to 71 at the beginning of the study and evaluated physical activity in relation to breast cancer diagnosis according to hormone-receptor status. Their results were consistent with the CTS, showing that higher levels of exercise decreased the risk of ER-negative breast cancer.
Other studies are needed to tell "whether the apparent protective effect of physical activity on breast cancer risk observed was due to a direct effect of exercise on hormone levels," says Dr. Michael Leitzmann, formerly of DCEG and lead investigator on the study. Other factors may also affect how exercise modulates breast cancer risk, including decreased levels of circulating insulin and insulin-like growth factors, reduction of chronic inflammation, and modulation of the immune response.
In addition to questions about mechanism, it's not yet clear how much exercise is needed for a protective effect, nor at what age exercise may have the greatest benefit on breast cancer risk. However, recent data from the Nurses' Health Study II (NHSII) suggest that beginning regular exercise early in adolescence and young adulthood may be important for the prevention of premenopausal breast cancer.
Investigators from the NHSII examined lifetime physical activity beginning at age 12 in 64,777 premenopausal women, and found a 23 percent reduction in risk for premenopausal breast cancer among women who exercised, on average, an equivalent of 3.25 hours a week of running or 13 hours a week of walking during adolescence. "Long-term, sustained activity showed the greatest benefit," says Dr. Graham Colditz, professor of medicine at Washington University in St. Louis and senior author of the study.
However, high levels of physical activity during ages 12 to 22 contributed most strongly to the observed reduction in risk in the NHSII study. Researchers speculate that this may have something to do with a vulnerable period in breast development. "From the time of early onset of puberty until first pregnancy, that's a time of maximal risk accumulation in terms of the breast not being fully developed, and that can be a period of great hormonal irregularity for many reasons beyond pubertal growth and maturation, including weight change, physical activity changes, dietary changes, and alcohol exposures," explains Dr. Rachel Ballard-Barbash, associate director of the Applied Research Program in NCI's Division of Cancer Control and Population Sciences.
"But most studies suggest that even if you weren't physically active at one period of life, that becoming physically active at any point in your life is beneficial," she continues. "It's just as important for women to be conscious of their overall health as it
is to be conscious of things that may have a specific influence on their breast health."
—Sharon Reynolds
|
 |
 |

FDA Takes Action on Ovarian Cancer Screening Test
The Food and Drug Administration (FDA) has issued a letter to Laboratory Corporation of America (LabCorp) saying the company lacks the legal authority to market its OvaSure test for the early detection of ovarian cancer. LabCorp rolled out the test last spring, shortly after results from a phase II clinical trial suggested the test is effective at both detecting disease early and identifying women who don't have it.
In the letter to LabCorp, Dr. Steven Gutman from the FDA's Center
for Devices and Radiological Health said an agency review of information about OvaSure "revealed serious regulatory problems involving these devices manufactured by
your firm."
The FDA typically does not require agency clearance of tests that will only be conducted at a single, central laboratory, as is the case with OvaSure. But tests have to be developed and manufactured by a single entity to receive such an exemption. OvaSure was developed by researchers from Yale University and includes components manufactured by other companies.
LabCorp began offering the test in June, specifically for use in women at high risk for the disease "to enhance the potential of detecting and treating ovarian cancer in its early or localized stage when the likelihood of survival is greatest," LabCorp Chief Medical Officer Dr. Myla Lai-Goldman said at the time. The 5-year survival rate in women for whom the disease is caught early, which happens in approximately one in five cases, is more than 90 percent. In women diagnosed with advanced disease, it drops to 30 percent.
Even before the FDA action, some concerns were expressed in the clinical community that OvaSure may have been introduced into commercial use prematurely. In July, for example, the Society of Gynecologic Oncology issued a position statement saying that "additional research is needed to validate the test's effectiveness before offering it to women outside the context of a research study."
The test is undergoing further evaluation in a phase III clinical trial in collaboration with NCI's Early Detection Research Network.
A LabCorp spokesperson told the Wall Street Journal's health blog that the company is "committed to working in partnership with the FDA to address the regulatory issues [raised in the agency's letter] and will provide an update as soon as we can."
|
 |
 |

Breast Density in Mammography and Cancer Risk
Studies from the last 3 decades have shown that breast density is directly linked to breast cancer risk. The magnitude of that risk is still under some debate, says Dr. Stephen Taplin, a senior scientist in NCI's Division of Cancer Control and Population Sciences who leads research on breast cancer screening as the project director for the Breast Cancer Surveillance Consortium (BCSC). But, he continues, "it is widely held to be one of the biggest risk factors, though the question of why it carries such a high risk is still not answered."
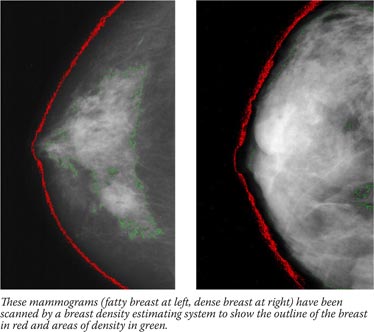
Breast density is not an intuitive concept, and has little to do with breast size. Breast density actually refers to the amount of white area on a breast that otherwise appears black on a mammogram. The balance of white and black reflects the breast composition and relative amount of glandular tissue, connective tissue, and fat. Different methods of estimating the proportion of white area on the mammogram exist and vary from the perception of the radiologist to using a software program to outline the white area and compare it to the total breast area.
Though it can be influenced by lifestyle factors, twin studies show that the underlying causes of breast density are mostly inherited. Higher breast density is more common in some ethnic groups, including White women. It is also more common in younger women, beginning when hormones kick in during puberty and continuing through the childbearing years.
Breast density decreases during menopause in a process called breast involution, where the milk-glands and ducts atrophy and connective tissue disappears. But in some women, these tissues persist into older age, and these are the women for whom the risk is a real concern, says Dr. Karla Kerlikowske, a professor of medicine and epidemiology and biostatistics at the University of California, San Francisco (UCSF) and principal investigator for the San Francisco BCSC research site.
"A 30-year-old woman can have dense breasts, but her underlying risk is still likely very low without other known risk factors," she explains. "We're really concerned with women who are in their 50s and 60s, who have persistent breast density, and who may benefit from a different screening procedure or some other preventive measure, such as chemoprevention."
To identify such women, Dr. Kerlikowske and colleagues in the BCSC published breast cancer risk prediction models in the Journal of the National Cancer Institute and the Annals of Internal Medicine. These models used the most common measurement for breast density, BI-RADS, to project cancer risk out to 1 and 5 years respectively for Caucasian, Asian, Hispanic, and Black women between the ages of 40 and 74, taking into account family history and breast biopsies.
The second model correctly identified women as low-risk and high-risk slightly better than the Gail model, but in the middle-risk group it was similar to the Gail model. The model hasn't been developed for clinical use, according to their paper.
The BI-RADS measuring system involves a radiologist scoring a mammogram in one of four categories according to the extent of contrast within the outline of the breast: x-rays pass easily through fatty tissue, which shows up as darker areas on the image, but are blocked - and thus appear white - by milk ducts, lobes, and the web of connective tissue that tethers everything together. Studies have shown that women who have extremely dense breasts have a three- to fivefold increased risk of breast cancer compared with women who have mostly fatty breasts.
One concern has been whether the breast cancer associated with dense breasts is due to a "masking effect," where the lack of contrast (that is, a breast image that is very white throughout) between normal tissue and tumors in dense breasts makes it difficult to discriminate between the two.
However, this isn't the only explanation for the relationship. "If we look at the risk association over time," says Dr. Taplin, "the longer out we go, the more evident it is that there's a fundamental risk factor that isn't about masking. It's something about the tissue itself."
 |
 |

Additional Imaging Technologies

Research on screening technologies, including digital mammograms, ultrasound, computer-assisted mammograms, and magnetic-resonance imaging, may also help address the dense-breast dilemma. However, these technologies are still being evaluated and the equipment in some cases is not yet widely available in clinics.

|
 |
|
|
What that could be isn't yet clear. In NCI's Division of Cancer Epidemiology and Genetics (DCEG), Drs. Mark Sherman, Louise Brinton, and Gretchen Gierach are looking for answers through the Breast Radiology Evaluation and Study of Tissues (BREAST) Stamp project, on which Dr. Taplin is also a collaborator. To date they have enrolled 179 women through the BCSC and will be examining tissues from breast biopsies and surgical resections to find markers that are associated with breast density and may determine whether epithelial cells develop into cancer precursors and cancer.
"Our working hypothesis is that the elevated risk associated with mammographic density reflects an altered microenvironment," explains Dr. Gierach, noting that they are looking closely at hormone levels and markers of inflammation. Another DCEG project led by Drs. Mark H. Greene and Jennifer Loud, the Breast Imaging Study, is examining how BRCA1 or BRCA2 gene mutations relate to breast density and includes a pilot analysis of the relationship between mammographic density, circulating estrogens, and estrogens within the breast itself.
Until there is an accurate and precise means to measure breast density, even though it is widely acknowledged as important in the scientific literature, it will not be a widespread consideration in the clinic, says Dr. Kerlikowske. "The reason is that we need a reliable measure in the clinic, and clinicians need to be trained to interpret risk information that includes a breast density measure."
She uses blood cholesterol and bone mineral density tests to illustrate the point. "When I order one of these tests on a woman, I get a printout that tells me what her score is, where she is in relation to women her age, and what I should do in terms of an intervention," she explains. "We need cost-effectiveness analyses to figure out the threshold for when a woman's breast cancer risk warrants clinical intervention - a different screening procedure, or chemoprevention, for example. Clinicians need to know the threshold of risk that requires an intervention."
They also need a measure of breast density that is more quantitative than the subjective BI-RADS system, she says. To this end, she and colleagues at UCSF are pilot-testing a new technology that calculates volumetric measurements of breast density using standard mammogram equipment. "That's where the future lies, and that's when you'll see breast density more widely used in the clinic," she says.
The conversation about breast density and cancer risk will principally be between researchers and clinicians until the issues surrounding density measurement and sub-groups of women who have dense breasts, and thus higher risk, get sorted out. But it isn't too early, some researchers advise, for women to discuss the matter with their doctors if they have concerns. This may be particularly important, says Dr. Taplin, for women who have persistent lumps but a negative mammogram.
—Brittany Moya del Pino
|
 |
 |
 NCI Staff Elected to IOM
NCI Staff Elected to IOM
Three NCI employees were recently honored with election to the Institute of Medicine (IOM): NCI Director Dr. John E. Niederhuber; Dr. Elaine Jaffe, chief of the Hematopathology section in the Center for Cancer Research's (CCR) Laboratory of Pathology; and Dr. W. Marston Linehan, chief of CCR's Urologic Oncology Branch. Their membership, among 65 new inductees, was announced at the IOM's annual meeting on October 13 at the National Academy of Sciences in Washington, D.C.
Membership in the IOM is considered one of the highest honors in the fields of health and medicine, and it recognizes individuals who have demonstrated outstanding professional achievements and commitment to service. Current active members elect new members from among candidates nominated for their accomplishments and contributions to the advancement of the medical sciences, health care, and public health.
Davis Named DTP Branch Chief
Dr. Myrtle A. Davis was recently appointed chief of the Developmental Therapeutics Program's Toxicology and Pharmacology Branch (TPB) in NCI's Division of Cancer Treatment and Diagnosis. Dr. Davis received her Ph.D. in toxicology from the University of Illinois at Champaign-Urbana and completed a post-doctoral fellowship in toxicologic pathology at the University of Maryland.
Before joining NCI, she worked at Eli Lilly Research Laboratories as a research advisor in toxicology in their Investigative Toxicology Group, where she focused on the development of kinase inhibitors as therapeutic agents. Prior to that, Dr. Davis was an associate professor in the Department of Pathology at the University of Maryland School of Medicine, where her research explored the mechanisms of toxin-induced apoptosis.
As branch chief of TPB, she serves as the toxicology expert for project and program teams in drug discovery through first human dose, provides mechanistic toxicology expertise, and creates and leads major research initiatives.
Understanding NCI Teleconference Series Continues
NCI's Office of Advocacy Relations will host its next "Understanding NCI" teleconference on "NCI's Cancer Information Service: Reaching the Community with Evidence-Based Resources" on October 29 from 2:00-3:00 p.m., ET. The featured speakers will be Ms. Mary Anne Bright, associate director of NCI's Office of Public Information and Resource Management; Ms. Lenora Johnson, director of NCI's Office of Communications and Education; and Ms. Carolyn Messner, director of education and training at CancerCare. A question and answer session for participants will follow their presentations.
No registration is required for participation. To join the teleconference, dial toll-free 800-857-6584; the passcode is: CIS. Toll-free playback will be available through November 29 at 800-216-6079. For more information about the "Understanding NCI" teleconference series and to learn about past teleconferences, go to: http://advocacy.cancer.gov/.
Breast Cancer Program-development Guidelines for Poorer Countries
The October 15 issue of Cancer includes a supplement developed by the Breast Health Global Initiative (BHGI) outlining how low- and middle-income countries can implement breast cancer programs to detect and treat the disease according to a tiered system of resource allocation based on a country's economic status and available resources. Although NCI does not necessarily endorse the recommendations, the supplement covers topics such as breast pathology, radiation treatment, surgery, and treatment of locally advanced cancer.
The NCI Office of International Affairs, headed by Dr. Joe Harford, is one of 19 international collaborating organizations of the BHGI, which works to advance the global fight against breast cancer.
| |
 |
 |
A Conversation with Steven Bognar and Julia Reichert
 Steven Bognar and Julia Reichert are filmmakers of the Emmy Award-winning documentary A Lion in the House, a film about the lives of five American families who each have a child battling cancer. Since its release in 2006, the film has been widely used by patient advocates and health agencies to promote greater understanding of the challenges facing pediatric patients and their families. It was recently released in a DVD edition.
Steven Bognar and Julia Reichert are filmmakers of the Emmy Award-winning documentary A Lion in the House, a film about the lives of five American families who each have a child battling cancer. Since its release in 2006, the film has been widely used by patient advocates and health agencies to promote greater understanding of the challenges facing pediatric patients and their families. It was recently released in a DVD edition.
What has been the response to the documentary, especially its use for education and advocacy?
We hoped A Lion in the House would have a cultural impact, particularly as a teaching tool. But the response has far exceeded our hopes. The movie has been used by many partner organizations to advocate for families fighting childhood cancer. It was shown in Congress to raise awareness of the need for the [Caroline Pryce Walker] Conquer Childhood Cancer Act, for example, and some of the families in the film spoke out there.
The DVD is packed with bonus features, including a short film updating viewers on all of the kids and their families. The film has been used in so many educational settings, we've lost count. At the same time, health care educators in many fields have asked for shorter clips for classroom use. So our team began - with major support from the Centers for Disease Control and Prevention, the Lance Armstrong Foundation, and SuperSibs! - tocreate from the film and its outtakes a series of targeted educational modules on selected subjects, include survivorship, health care disparities, end-of-life communication, school issues, nurse boundaries, sibling issues, and spirituality issues. Each mini-movie module comes with unique learning objectives, discussion questions, competencies, take-home points, and an in-depth resource list. The educational materials can be purchased on the Web at http://www.aquariusproductions.com.
The modules have been designed with input from a team of top experts in their respective fields, who looked at draft after draft to help us refine and improve them. They are for many audiences - medical and nursing students, residents, fellows, nurses, social workers, and others.
The module on disparities has been used to advocate for transportation support for poor families, and the school issues module has been widely used to educate school nurses and counselors on childhood cancer's impact on learning, for both children with cancer and their siblings. We are extremely gratified that many organizations in our field have embraced the film and the related modules and have found creative ways to use them.
How has your involvement in making the film affected your lives and work?
A Lion in the House changed our lives profoundly. Making the film deepened us as people and gave us a greater sense of compassion for all of our fellow human beings. We saw intimately the depth of love that exists between parent and child. The world of cancer treatment, perhaps especially childhood cancer, is populated by a particular breed of doctors, nurses, social workers, and others. We came into close contact with professional caregivers, whose dedication and sensitivity leave a lasting impression. It was the hardest film we ever made and the most gratifying.
What can potential filmmakers or advocates do if they are considering approaching a subject like this?
Be ready for the long haul. Be open to deep emotion and to the stories of others. Get to know the organizations that have been fighting for awareness of the needs of children and families facing cancer and other life threatening illnesses. Meet them face-to-face. Learn their goals and actions. It's actually a small community, full of really great people. But you'll have to be tireless and patient in finding them all and connecting.
|
 |
|