| |
| Neuroscience. Author manuscript; available in PMC 2006 October 12. Published in final edited form as: doi: 10.1016/j.neuroscience.2005.05.057. | PMCID: PMC1599838 NIHMSID: NIHMS12533 |
Differential Expression of Arc mRNA and Other Plasticity-Related Genes Induced by Nicotine in Adolescent Rat Forebrain T. L. SCHOCHET,a A. E. KELLEY,b and C. F. LANDRYb* aNeuroscience Training Program, University of Wisconsin-Madison, 6001 Research Park Boulevard, Madison, WI 53719, USA bDepartment of Psychiatry, University of Wisconsin-Madison, 6001 Research Park Boulevard, Madison, WI 53719, USA |
Abstract Relatively little attention has been focused on mechanisms related to neural plasticity and drug abuse in adolescence, compared with abundant research using adult animal models. As smoking is typically initiated in adolescence, an important question to address is whether the adolescent brain responds differently to nicotine compared with the adult. To investigate this question, we examined the expression of a number of early response genes (arc, c-fos and NGFI-B) that have been implicated in synaptic plasticity and addiction, following acute nicotine in adolescent and adult rats. Baseline expression of arc and c-fos was higher in adolescent brains compared with adults. Following acute nicotine treatment (0.1, 0.4 mg/kg), we found a marked induction of arc mRNA in the prefrontal cortex of nicotine-treated adolescents compared with a less pronounced increase of arc in the adult. c-fos and NGFI-B were also upregulated by nicotine, but not in an age-related manner. In contrast, nicotine induced less arc, c-fos, and NGFI-B expression in the somatosensory cortex of adolescents compared with adults. A fourth gene, quinoid dihydropteridine reductase was expressed at lower levels in white matter of the adolescent forebrain compared with the adult, but was not affected by nicotine. These results suggest that in adolescence, the activity of specific early response genes is higher in brain regions critical for emotional regulation and decision-making. Further, nicotine affects key plasticity molecules in these areas in a manner different from the adult. Thus, adolescence may represent a neurobiologically vulnerable period with regard to nicotine exposure. Keywords: plasticity, development, drug abuse, prefrontal cortex, immediate-early gene |
Smoking is an addictive habit that typically develops in adolescence. Despite its clear clinical relevance, relatively little is known about the neurobiology of immediate and long-term consequences of smoking during adolescence, or age-specific contributions to nicotine addiction. Nicotine has a number of well-established neurochemical and molecular effects on adult neural systems ( Dani and Heinemann, 1996), including upregulation of nicotinic acetylcholine receptors (nAChRs) following chronic nicotine treatment ( Wonnacott, 1990; Perry et al., 1999). However, beyond central cholinergic effects, systems involved in cellular plasticity and learning are also markedly affected by nicotine. Nicotine has been linked to increased dopamine levels in mesocortical limbic regions ( Di Chiara and Imperato, 1988), and its behavioral and rewarding effects are partially dependent on dopaminergic activation ( Clarke et al., 1988; Di Chiara, 2000). Nicotine also interacts with glutamate systems in the brain, enhancing fast excitatory synaptic transmission at glutamatergic synapses ( McGehee et al., 1995). Since current major theories of addiction and memory implicate interactions between dopaminergic and glutamatergic systems, this profile of nicotine effects suggests nicotine may induce long-term synaptic alterations at the level of gene expression. Indeed, in the adult rat model, studies have shown acute and chronic nicotine administration activates the immediate-early gene c-fos in multiple limbic and cortical regions ( Nisell et al., 1997; Pich et al., 1997; Salminen et al., 1999). Other immediate early genes implicated in the mesocorticolimbic response to nicotine include cAMP-response element CREB and deltaFosB ( Kelz et al., 1999; Pandey et al., 2001). The extent to which neurochemical and molecular mechanisms identified as important in the adult are applicable to the adolescent brain is undetermined. Adolescent rats display altered behavioral responses to nicotine compared with adults in a number of paradigms ( Vastola et al., 2002; Faraday et al., 2003; Levin et al., 2003; Belluzzi et al., 2004; O’Dell et al., 2004). We have recently reported that although the overall locomotor doseresponse sensitivity to nicotine is similar in adolescents and adults, adolescent rats fail to display long-term contextual cue conditioning ( Schochet et al., 2004). Changes in nicotinic acetylcholine, serotonergic, dopaminergic and glutamatergic receptor systems in forebrain and midbrain regions following prolonged adolescent nicotine exposure have all been reported ( Slotkin, 2002; Abreu-Villaca et al.,2003a, 2003b; Ad-riani et al., 2003; Collins et al., 2004b). Relatively little is known about the effect of nicotine on plasticity-related genes in the adolescent brain. As cortical substrates are actively developing in the adolescent ( Lewis, 1997), we hypothesized that nicotine might differentially affect expression of these genes in adolescents compared with adults. We report here that arc, NGFI-B, and c-fos, three plasticity-related early response genes, are upregulated in cortical and striatal sites following acute nicotine. Moreover, the expression of arc, a dendritically targeted mRNA whose protein product is involved in synaptic modification and learning, is more strongly induced by nicotine in adolescence than in adulthood. |
Subjects and handling For all experiments, male Sprague-Dawley rats (Harlan, Madison, WI, USA) were used. Animals were housed in pairs in clear plastic cages in an animal colony. Food and water was available at all times. Lighting in the animal colony was on a 12-h light/dark cycle, with lights on at 07:00-19:00 h. Rats arrived in the colony 4 days prior to testing, and were handled daily in order to minimize stress during testing. All animal care was in strict accordance with University of Wisconsin-Madison Institutional Animal Care and Use Committee guidelines as defined by the NIH. Care was taken to minimize the number of animals used and their suffering. The effects of nicotine on gene expression were assessed in two separate conditions. Our first experiment examined the acute effects of nicotine and the second experiment examined the doseresponsivity of the gene expression. For the examination of the acute nicotine effects on gene expression, we used a total of 32 rats. Of these rats, 16 were tested at approximately 70 days of age (adult, average weight 306 g), and 16 were tested at 30 days (early adolescent, average weight 84 g). On the test day, rats were given a single s.c. injection of nicotine [ n=8 adolescent, n=8 adult, 0.4 mg/ml/kg s.c. nicotine hydrogen tartrate salt (Sigma, St. Louis, MO, USA), dissolved in saline, pH adjusted to 7.2 with NaOH] or a single saline injection ( n=8 adolescent, n=8 adult, 1 ml/kg, s.c.). We have previously found that adolescent and adult animals show similar locomotor responses to increasing doses of nicotine. The moderate dose of 0.4 mg/kg nicotine used in our previous sensitization and conditioning experiments was used in this study ( Schochet et al., 2004). In our second experiment, we examined whether the effect of nicotine on arc expression in adolescents and adults was dose-dependent using a total of 16 rats. Of these rats, eight were tested at approximately 70 days of age (adult, average weight 303 g) and eight were tested at 30 days (early adolescent, average weight 99 g). On the test day, rats were given a single s.c. injection of nicotine (n=4 adolescent, n=4 adult, 0.1 mg/ml/kg s.c. nicotine hydrogen tartrate salt (Sigma, St. Louis, MO, USA), dissolved in saline, pH adjusted to 7.2 with NaOH) or a single saline injection (n=4 adolescent, n=4 adult, 1 ml/kg, s.c.). In all studies, injections were administered between 12:00 and 14:00 h. One hour after the injection, rats were anesthetized with halothane, and their brains rapidly removed, frozen, and stored at -80 °C. Cryostat sections (20 μm) were collected onto Superfrost plus microscope slides (Fisher, Pittsburgh, PA, USA) and stored dry at -80 °C until use. In situhybridization Sections were post-fixed in 4% paraformaldehyde for one 1/2 h at 4 °C. Following three washes of 2× SSC (1× SCC=150 mM NaCl, 15 mM citrate, pH 7.0), slides were briefly digested with Proteinase K (0.2 μg/μL; Qiagen, Valencia, CA, USA) for 10 min at 37 °C. Slides were then acetylated for 10 min at room temperature with 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8, washed in 2× SSC and dehydrated in a graded ethanol series. Sections were hybridized overnight at 55 °C in a hybridization solution containing 10% dextran sulfate, 3× SSC, 0.5 M NaPO 4, 50% formamide, 1× Denhart’s, and 200 μg/ml tRNA, pH 7.5, 0.05 M DTT and 0.1 ng/μL[ 35S]-labeled antisense cRNA probe. Following hybridization, sections were washed in 500 mM NaCl, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5, and digested for 1h at 37 °C in the same solution containing 20 μg/ml pancreatic RNAse A (Ambion, Austin, TX, USA). Slides were then washed in 1× SSC, 0.2 M DTT (5 min), 0.5× SSC, 0.2 M DTT (5 min), and 0.1 M SSC, 0.2 M DTT (1 h, 70 °C), dehydrated in a graded ethanol series and dried. For autoradiography, sections were exposed to a phosphorimager screen for one week and scanned with a Molecular Dynamics Typhoon phosphorimager (Molecular Dynamics Inc., Sunnyvale, CA, USA). Slides were also subjected to autoradiography for 6 weeks using Kodak NTB-2 liquid emulsion (Eastman Kodak Co, Rochester, NY, USA) and stained as previously described ( Landry et al., 1989). Photomicrographs were taken on a Leica DMRX microscope equipped with a Leica DC300F camera. Probe preparation Four genes were chosen for analysis, arc, c-fos, NGFI-B and quinoid dihydropteridine reductase ( QDR). Arc, c-fos, and NGFI-B are immediate early genes selected for study based on their role in synaptic plasticity and sensitivity to drugs of abuse. Increases in arc, c-fos, and NGFI-B expression have been described following treatment with drugs including cocaine, morphine, amphetamine and nicotine ( Fosnaugh et al., 1995; Konradi et al., 1996; Pich et al., 1997; Werme et al., 2000a; Steward and Worley, 2002). We previously found that the gene QDR ( Turner et al., 1974) was expressed at higher levels in the adult compared with the adolescent brain based on preliminary gene expression microarray analysis ( Schochet et al., 2002). Total RNA from rat brain was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and used to generate a cDNA library using reverse transcriptase as described by the manufacturer (Amersham Biosciences, UK). cDNA for arc, NGFI-B and QDR used for in situ hybridization was amplified from this library using standard PCR conditions. The following primers were used to generate PCR products for the gene indicated. Numbers in parentheses after the primer sequence represent the base number as defined by the gene sequence in the Unigene database. The sequence in italics corresponds to the T7 polymerase recognition sequence used for 35S-labeled antisense cRNA probe generation as described by the manufacturer (Promega Corporation, Madison, WI, USA). Arc, forward primer 5-CCCCAGGAAGCTGATGGCTAC-GAC-3′(693-714), reverse primer ′ 5′-CAGAGATGCATAATAC-GACTCACTATAGGGAGAGAGTGTCAGCCCCAGCTCAATCA-AG-3′(1472-1494). NGFI-B, forward primer 5-GGTGTATGGCTGCTACCC-TGG-3′(428-448), reverse primer ′ 5′-CAGAGATGCATAAT-ACGACTCACTATAGGGAGAGAGTCCAAATGTGCTCGAATG-AGG-3′(1208-1229). QDR, forward primer 5-TCAGTTCCGCGGGAGTCTT-3 (20-39), reverse primer 5-CAGAGATGCATAATACGACTCACTAT-′ AGGGAGAGATTACAGGCCCCCACTCATTC-3 ′ (970-950). c-fos, forward primer 5′-AATAAGATGGCTGCAGCCAA-′ 3′(573-592), reverse primer 5′-CAGAGATGCATAATACGACT-CACTATAGGGAGAGAGGATGGCTTGGGCTCAAGGT-3′ (890-871). Plasmid pBluescript-II-SK containing c-fos cDNA was kindly provided by T. Curran. Plasmid was linearized with BamH1 and transcribed with T7 polymerase for the generation of 35S-labeled antisense cRNA probe [573-890]. Data analysis Optical density data values were generated using the ImageQuant software (Molecular Dynamics, Amersham Biosciences). For arc, c-fos, and NGFI-B, six brain regions were chosen for analysis, based on data in adult rats showing their involvement in the overall drug response. These regions were the medial prefrontal cortex (mPFC), ventral and lateral orbital cortex (VLO), cingulate cortex, somatosensory cortex (SmCtx), ventral striatum (VS), and dorsal striatum (DS). As shown in Fig. 1, analysis was performed by delineating standard geometric shapes around the appropriate anatomical region. One section was analyzed per brain region, and counting was carried out on both sides of each chosen section. Following automatic calculation, density values were subsequently normalized to white matter density values calculated for each slice. This normalization was performed to adjust for differential binding of probe to each section; as these genes are not expressed in white matter, the levels of intensity in these regions would represent background nonspecific binding. For QDR, a gene found primarily in white matter, expression levels were measured in the forceps minor of the corpus callosum (fmi), the genu of the corpus callosum (gcc) and the nucleus of the vertical limb of the diagonal band (VDB). A region of gray matter not expressing QDR was selected for each section to provide background binding values. 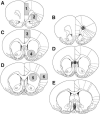 | Fig. 1Schematic diagrams of brain regions selected for gene expression analysis. Coronal forebrain sections were subjected to in situ hybridization and the numbered regions further analyzed using densitometry as described in Experimental Procedures. The light (more ...) |
Two kinds of statistical analysis were carried out on the in situ hybridization data. To determine cortical versus subcortical effects, a three-factor, between-within ANOVA was carried out with treatment and age as between-subjects factors, and brain region (cortical vs. subcortical) as the within-subjects factor. The cortical regions were analyzed by compacting optical density values for mPFC, VLO, cingulate and SmCtx as one variable. Subcortical or striatal regions were composed of the optical density values for VS and DS compacted as one variable. If overall interactions indicated significance, a two-factor ANOVA was performed with treatment and age as the between-subjects factors for each individual brain region. Cell counts of arc mRNA-expressing cells in the VLO were performed on images collected on a Leica DMRX microscope equipped with a Leica DC300F camera. Silver grain accumulations corresponding to arc-expressing cells were visualized in dark field at 20× objective magnification and counted using Image Pro Plus 5.0.0.39. Two animals in each of four groups (adolescent saline and nicotine, adult saline and nicotine) were analyzed and data from the VLO from each hemisphere were pooled ( N per group=4). An area of 0.55 mm by 0.68 mm corresponding to the boxed region shown in Fig. 4A was counted and a size cutoff of 100 um 2 was employed to eliminate individual and non-specific clusters of silver grains from contributing to the total count.  | Fig. 4Arc mRNA accumulation in adolescent cortex following nicotine administration occurs primarily in layers IV to VI. (A) Representative cortical section from a saline-treated adolescent rat following in situ hybridization and emulsion autoradioagraphy showing (more ...) |
|
Arc mRNA is differentially expressed in specific regions of the prefrontal cortex Gene expression profiling using in situ hybridization was used to determine whether acute nicotine treatment differentially affects the expression of early response, plasticityrelated genes in the adolescent compared with the adult forebrain. One of the genes that was selected for this analysis, the activity regulated, cytoskeletal-associated gene arc, undergoes dynamic changes in dendritic mRNA localization in response to specific stimuli in the hippocampus and is important in long-term potentiation ( Steward and Worley, 2002). Following acute nicotine treatment at a dose of 0.4 mg/kg, an increase in arc expression was evident in specific forebrain regions of both the adolescent and adult compared with saline controls ( Fig. 2B). In regions of the adolescent cortex, however, this nicotine-induced increase was much greater than that observed in the adult, particularly in prefrontal and sensorimotor cortical regions ( Fig. 2B). However, baseline (saline treated) arc mRNA expression was found to be higher in the adolescent compared with the adult brain. This baseline difference may be linked to alterations in active synaptogenesis in the adolescent brain, as arc expression in dendritic spines has been correlated to synaptogenesis in opioidresponsive neurons in rat caudate-putamen ( Wang and Pickel, 2004). To determine whether the induction of arc in specific cortical regions was due to a differential increase over baseline levels, we used densitometry to compare arc expression levels between regions and ages. 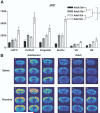 | Fig. 2Arc expression is differentially induced in specific regions of the adolescent forebrain following acute nicotine (Nic) administration at 0.4 mg/kg. (A) Regions of the adolescent and adult forebrain were analyzed using densitometry after sections were (more ...) |
Statistical analysis confirmed the enhanced nicotine-induced increase in arc expression observed in adolescent cortical regions ( Fig. 2A). A significant age×treatment interaction was present [ F(1,20)=4.853, P=0.0395] for arc in cortical, but not striatal regions, indicating a differential effect of nicotine on arc expression in adolescent animals. ANOVA analysis of cortical regions further indicated significant main effects of age [ F(1,20)=31.210, P<0.001], treatment [ F(1,20)=28.490, P<0.001], and region [ F(3,30)= 8.881, P<0.001]. Significant main effects were also present in the subcortical (striatal) regions for age [ F(1,20)=17.613, P<0.001], treatment [ F(1,20)=6.525, P=0.05], and region [ F(1,20)=11.887, P<0.01]). This overall profile as well as significant age×region interactions in both the cortical [ F(3,60)=2.797, P>0.05) and subcortical analyses ( F(3,60)=10.841, P>0.005) indicates that the brains of adolescent animals expressed more arc mRNA overall. A more focused ANOVA on select brain areas was performed to further determine region-specific effects of nicotine on arc expression. Most strikingly, a significant age×treatment interaction was present in the VLO cortex [F(1,20)=4.932, P=0.0381] indicating a differential induction of arc mRNA in this region of the adolescent prefrontal cortex compared with the adult. In the VLO, adolescent rats given nicotine displayed a 182% increase in arc expression relative to their saline counterparts compared with a 98% increase in adults given nicotine compared with adults given saline. Trends toward a significant age×treatment interaction were also present in the adolescent medial prefrontal [F(1,20)=2.684, P=0.1170] and sensorimotor cortex [F(1,20)=3.939, P=0.0611], suggesting that the differential induction of arc expression in the adolescent may be present in a number of cortical regions. In the mPFC, arc expression in nicotine-treated adolescents was increased 114% from saline treatment, whereas adult nicotine treatment values were 53% higher than saline counterparts. Interestingly, in sensorimotor cortex, adolescents expressed arc only 130% more when given nicotine compared with saline, whereas adults given nicotine expressed 229% more arc than saline-treated animals. Significant main age effects were present in all regions examined, indicating increased arc expression in adolescent brain occurred in a global manner. Significant main effects of treatment for both adolescents and adults were present for all regions except DS, indicating that arc expression increased after nicotine administration. A lower dose of nicotine, 0.1 mg/kg, was tested to determine if the observed increases in arc expression were dose dependent. At this dose of nicotine, no age×treatment effects were present, although adolescents again expressed more arc overall [ F(1,88)=29.342, P<0.001], and nicotine tended to increase arc mRNA expression [ F(1,88)=3.784, P=0.05] ( Fig. 3), confirming the previous experiment. This suggests that the differential expression of arc observed at 0.4 mg/kg was not due merely to a general increased sensitivity of adolescents to nicotine.  | Fig. 3Arc expression is not differentially induced following acute administration at 0.1 mg/kg. Bar graph of densitometry data showing signal density in specific forebrain regions. Nic, nicotine, Sal, saline; LO, lateral orbital cortex; * treatment effect, (more ...) |
The elevation in arc expression in the adolescent cortex following nicotine administration occurred in a layer-specific pattern ( Fig. 4). In saline-treated adolescents, arc expression was primarily evident within the deeper layers of the cortex. An example of this expression pattern in S1 region of the frontal cortex is shown in Fig. 4A. Following nicotine treatment, in addition to an accumulation of arc mRNA within the deeper cortical layers, a dramatic elevation in arc mRNA was evident within layer IV ( Fig. 4B). High magnification micrographs of sections subjected to arc in situ hybridization and emulsion autoradiography indicated that silver grain accumulation occurred primarily over cells containing large, pale-staining nuclei, consistent with labeling over cells of a neuronal morphology (arrows in Fig. 5). An example of a typical labeling pattern in adult and adolescent cortex is shown for the VLO ( Fig. 5), a region where a differential induction of arc in adolescents relative to adults was evident. Although basal levels of arc in the VLO were higher in the adolescent than the adult (compare Fig. 5C and D), a greater nicotine-induced increase in arc was present in the adolescent ( Fig. 5E) compared with the adult ( Fig. 5F).  | Fig. 5Nicotine induces arc expression in neurons of the ventrolateral orbital cortex. (A) Coronal forebrain sections were analyzed for optical density on a phosphorimager and autoradiography using liquid emulsion. Boxed area represents the regions of the VLO (more ...) |
Cell counts of the VLO were performed to determine whether the increase in arc expression following nicotine administration was due to an increase in the number of cells expressing arc or an increase in arc expression within cells that express the mRNA in the basal state. We found that the number of arc mRNA-expressing cells in VLO was similar in adults and adolescents and did not change after nicotine treatment (adolescent nicotine, 211±33; adolescent saline, 197±23; adult nicotine, 180±15; adult saline, 191±9) suggesting that arc expression increased in cells that expressed arc in the basal condition. Fos mRNA is induced in adolescent forebrain following nicotine treatment We also examined the influence of acute nicotine treatment on the expression of the early response transcription factor, c-fos. Interestingly, c-fos expression patterns following nicotine treatment in both the adolescent and adult forebrain were similar to the expression patterns observed for arc (compare Fig. 6B with 2B). As with arc expression, the highest levels of c-fos were observed in adolescent VLO and SmCtx ( Fig. 6A). However, no significant age×treatment interactions were found for cortex, indicating that nicotine increases c-fos expression to the same degree in adolescents as in adults. Statistical analysis of cortical regions indicated significant main effects of age [ F(1,20)=14.999, P<0.001], treatment [ F(1,20)=21.648, P<0.001], and region [ F(3,60)=26.878, P<0.001]. Significant main effects were also present in the subcortical (striatal) regions for age [ F(1,20)=17.613, P<0.001], treatment [ F(1,20)=13.867, P=0.001], and region [ F(1,20)= 5.919, P<0.05). However, a significant age×region interaction was present only for cortex [ F(3,20)=5.067, P>0.05], as the adolescents displayed greater cortical expression of c-fos, suggestive of altered plasticity mechanisms in these areas. 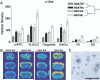 | Fig. 6c-fos Is induced in the adolescent and adult forebrain following acute nicotine (Nic) administration. (A) Bar graph of densitometry data showing signal density in specific forebrain regions. (B) Representative sections from adolescent and adult animals (more ...) |
The more focused ANOVA indicated that significant main effects of age and treatment were present in all regions, with the exception of DS, in which there were no significant treatment effects. In contrast with the effects of nicotine on arc expression, no significant interactions were present for any regions, although a strong trend toward a significant age×treatment interaction was present in the VS [ F(1,20)=4.210, P=0.0535)], where acute nicotine appeared to cause a larger increase in c-fos mRNA levels in the adolescent than in the adult. High magnification micrographs of sections subjected to emulsion autoradiography again indicated that silver grain accumulation occurred primarily over cells containing large, pale-staining nuclei, consistent with labeling over cells of a neuronal morphology ( Fig. 5C). We found no difference in c-fos mRNA-expressing cells in VLO between adults and adolescents and no change after nicotine treatment. A similar induction of NGFI-B is evident in adolescent and adult forebrain after acute nicotine NGFI-B is an immediate early gene encoding an orphan nuclear receptor that is rapidly recruited under a variety of stimuli ( Svenningsson et al., 1995; Werme et al.,2000a, 2000b), and we therefore examined the expression of this gene following nicotine administration. Unlike the pattern of expression seen for arc and c-fos, acute nicotine did not produce any differential age effects for NGFI-B, although nicotine treatment generally increased NGFI-B expression ( Fig. 7). The overall analysis of NGFI-B revealed significant main effects of treatment in both cortical [ F(1,20)= 5.087, P>0.05] and subcortical regions [ F(1,20)=4.463, P>0.05]. Main effects of region were also present for both cortex [ F(3,60)=11.598, P<0.001] and striatum [ F(3,60)=33.055, P>0.001]. However, no main effects of age were present. A significant treatment×region interaction was found for cortex [ F(3,60)=6.476, P>0.001], but not striatum, suggesting that nicotine affected NGFI-B expression more strongly in cortical regions than subcortical. Conversely, a significant age×region interaction was present in the striatum [ F(3.60)=9.942, P=0.005] but not cortex. In Fig. 7, it is apparent that the overall levels of NGFI-B expressed in the DS may be higher in adolescents than adults. Silver grain accumulations in emulsion-treated slides were similar to arc and c-fos suggesting expression of NGFI-B in neurons ( Fig. 7c). 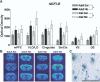 | Fig. 7NGFI-B mRNA expression was elevated in the forebrain of adolescent and adult rats following nicotine (Nic) treatment. (A) Bar graph of optical density scans showing a moderate induction of NGFI-B in specific forebrain regions. (B) Coronal forebrain sections (more ...) |
QDR expression is not affected by acute nicotine treatment We were interested in determining whether acute nicotine administration affected the expression of QDR, an enzyme implicated in monoamine and nitric oxide biosynthesis ( Turner et al., 1974; Gorren and Mayer, 2002). In a preliminary microarray analysis directed at identifying genes whose expression patterns differed in the adolescent compared with the adult brain, QDR was found to be expressed at higher levels in adult brain ( Schochet et al., 2002). Further, QDR was present primarily in white matter and was expressed in oligodendrocytes (not shown). An examination of major white matter regions following saline or nicotine treatment did not reveal any effect on QDR expression ( Fig. 8A), although intense expression of QDR was evident in the adult forebrain ( Fig. 8B). Therefore, QDR served as a non-affected “control” gene for our analysis. 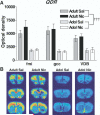 | Fig. 8QDR is expressed at higher levels in adult compare with adolescent forebrain. (A) Optical density bar graphs of specific white matter regions from adolescent and adult rats treated with either saline (Sal) or nicotine (Nic). Although Nic had no affect (more ...) |
|
These studies report three novel observations. First, the expression of arc, a dendritically targeted gene important for synaptic plasticity and involved in learning and memory, is upregulated in adolescence, and moreover, differentially increased in specific cortical regions of the adolescent compared with the adult following acute nicotine administration. Second, the plasticity-related genes arc and c-fos, but not NGFI-B, are higher in the baseline state in adolescent forebrain compared with adults. Third, the oligodendrocyte-enriched gene QDR is markedly more abundant in the adult compared with the adolescent brain, although its expression is not affected by nicotine. Taken together, these results suggest that a dynamic developmental profile of the expression of specific molecular markers is present in adolescent brain, and that acute nicotine influences the expression of plasticityassociated markers. Maturation of the adolescent brain The prefrontal cortex, an area of adolescent brain where we found differential induction of arc following acute nicotine, undergoes dramatic changes during adolescence including extensive synaptic pruning, alterations in dopaminergic input and changes in intrinsic circuitry ( Rosenberg and Lewis, 1995; Cunningham et al., 2002; Erickson and Lewis, 2002; Cruz et al., 2003). Anatomical tracing studies indicate that projections from the amygdala to mPFC continue to increase in density throughout adolescence ( Cunningham et al., 2002), suggesting that connectivity between emotional and cognitive areas undergoes refinement during this period. Further, cortical gray matter volume changes dynamically during adolescence in areas including mPFC and orbital prefrontal cortex ( Kolb and Nonneman, 1976; Seeman et al., 1987; Giedd, 1999; Giedd et al., 1999; Seeman, 1999). Similarly, cerebral white matter volume increases throughout the adolescent period ( Giedd et al.,1996a, 1996b) in parallel with myelination of intrinsic fiber connections ( Nauta, 1971; Benes, 1989; Paus et al., 1999). These findings suggest that plastic changes occur within the mammalian forebrain throughout the adolescent period. Basal and nicotine-induced acute arc expression levels in the brain are age-dependent Arc appears to be part of this developing substrate. Arc is an immediate-early gene that marks synapses undergoing modification ( Lyford et al., 1995), is implicated in activitydependent plasticity and memory ( Steward et al., 1998; Steward and Worley, 2002; Kelly and Deadwyler, 2003) and accumulates in dendrites at sites of recent synaptic activity ( Guzowski et al., 2000; Guzowski, 2002). Additionally, arc is upregulated following amphetamine and cocaine administration in a number of brain regions ( Fosnaugh et al., 1995; Yamagata et al., 2000). For the first time, we show that arc is also strongly induced by nicotine in adult and adolescent rats in a dose-dependent manner, suggesting that the effects of nicotine involve alterations in genes and proteins regulating the post-synaptic density. Higher basal and drug-induced expression of arc in the adolescent suggests that altered underlying cortical plasticity reflective of an active state of synaptic modeling is present in the forebrain during this stage of development. Within the cortex, nicotine altered the distribution of arc expression in a layer-specific manner. Arc expression was higher in the adolescent than the adult in cortical layer VI, a cortical output layer. Following acute nicotine, layer IV, which receives heavy input from the thalamus, was strongly recruited ( Guillery and Sherman, 2002). Thus, this profile of increased arc expression suggests that nicotine has a profound effect on plasticity in integrative thalamocortical networks. Differential induction of arc in the nicotine-treated adolescent was greatest in the ventrolateral orbital region of the prefrontal cortex, an area of brain important for maintaining representations of reward value and in guiding goal-directed responses ( Pickens et al., 2003; Rolls, 2004). The occurrence of nicotine-induced gene induction primarily in cortical rather than striatal regions perhaps reflects a preferential effect of nicotine on cognitive and attentional functions, which may be immature in the adolescent ( Benes et al., 2000). Immaturity of systems involved in reward and cognition is implied by recent electrophysiological data showing NMDA-D1 enhancement of depolarized “up states” in prefrontal cortex is not present in early adolescence and develops only in adulthood ( Tseng and O’Donnell, 2005). In addition, a less pronounced effect of nicotine on arc expression in adolescent striatum may be due to differences in relative maturity of this region compared with cortical regions, as in fact, striatal neurons reach morphological and electrophysiological maturityprior to adolescence ( Tepper and Trent, 1993). Given that arc is known to be regulated by NMDA and dopamine receptors, the upregulation of arc induced by nicotine may be mediated by these receptor systems. Additionally, given the well-established effects of nicotine on cholinergic function, a role for nicotinic receptors in the gene response to nicotine should not be excluded. Indeed, upregulation of nicotinic receptor subtypes and increases in cholinergic receptor binding have been reported in midbrain, cortex and hippocampus following adolescent nicotine exposure ( Trauth et al., 1999; Adriani et al., 2004). Adolescent nicotine exposure also increases nicotine self-administration in adulthood ( Adriani et al., 2004). As our analysis only considered forebrain regions, other age-related effects may be present in additional brain areas. In fact, in contrast to more rostral brain regions, induction of arc, c-fos and NGFI-B in SmCtx was less pronounced in adolescents given nicotine than in adults. These results are consistent with recent studies showing that the time-course of the development of nicotine-induced c-fos expression varies in different sensory cortical and limbic regions ( Leslie et al., 2004). It will be interesting to determine whether other brain regions not examined in the current study, such as the hippocampus and amygdala, show similar differences in nicotine-induced early response gene expression between adolescents and adults. The possibility that plasticity mechanisms with regard to drug exposure are different in adult and adolescent brains is intriguing, given there are differences in the behavioral response to nicotine and other drugs of abuse in adolescent and adult rats ( Spear, 2000; Vastola et al., 2002; Belluzzi et al., 2004). For example, adolescent rats show blunted locomotor sensitization to repeated nicotine, compared with adults, and reduced cue-conditioning ( Collins et al., 2004a; Schochet et al., 2004). In fact, the lack of nicotine cue conditioning in the adolescent compared with the adult may relate to ceiling levels of arc expression in the prefrontal cortex, a region critical for expression of contextual conditioning ( Schroeder et al., 2001; Schiltz et al., 2003; Schochet et al., 2004). The immediate early genes c-fos and NGFI-B are upregulated by nicotine, but not in an age-dependent manner c-fos, a well-characterized transcription factor, was induced in the adolescent brain following acute nicotine. Consistent with these data, age-dependent effects have been demonstrated for c-fos, which has been shown to progressively increase in the prefrontal cortex and striatum during the adolescent period ( Kellogg et al., 1998; Andersen et al., 2001; Leslie et al., 2004). Since higher levels of c-fos mRNA were evident in the adolescent fore-brain following saline treatment, it is likely that the threshold for induction of this gene is lower in the adolescent brain than in the adult, suggesting that basal neuronal activity may be higher in the adolescent. Nicotine and other drugs of abuse increase c-fos expression under D1 and NMDA receptor control ( Pagliusi et al., 1996; Nisell et al., 1997; Pich et al., 1997; Ostrander et al., 2003) and can be blocked by dopamine and glutamate antagonists ( Kiba and Jayaraman, 1994; Konradi et al., 1996; Liu and Weiss, 2002). An induction of NGFI-B ( Nur77) following acute nicotine administration has not been previously reported. NGFI-B is an orphan nuclear receptor belonging to a larger class of steroid-thyroid hormone receptors and has been associated with dopaminergic target structures ( Zetterstrom et al., 1996). Chronic morphine or cocaine administration, as well as other manipulations, alters the expression of NGFI-B in cortex, striatum, and accumbens ( Svenningsson et al., 1995; Werme et al.,2000a, 2000b). Of the three early-response genes studied, NGFI-B was the only gene whose expression pattern was the same in adolescents and adults regardless of treatment, indicating it is less influenced by a developmental context. Taken as a whole, the observation of changes in arc and c-fos, but not NGFI-B expression, suggests that the downstream targets of arc and c-fos may be more affected by adolescent development than targets of NGFI-B. QDR is expressed in an age-dependent manner, but is not affected by nicotine administration QDR was expressed at higher levels in the adult compared with the adolescent brain in a distribution suggesting principal expression in white matter (oligodendrocytes). QDR is required in the regeneration of tetrahydrobiopterin, a cofactor essential for the function of aromatic amino acid hydroxylases and nitric oxide synthases, implicating the enzyme in several important biosynthetic pathways ( Turner et al., 1974; Kaufman et al., 1975; Gorren and Mayer, 2002). Our observation that QDR is expressed primarily in oligodendrocytes suggests age-related differences in biosynthetic mechanisms that may be related to differences in white matter volume and myelination observed in the adolescent. |
These results have special relevance to the problem of nicotine addiction, a major contributor to morbidity and mortality in this country. Although the precise underlying causes and substrates of addiction are unknown, a major theory posits that addictive drugs interact with and influence primary motivational systems, and in particular the plasticity mechanisms within these systems ( Jentsch and Taylor, 1999). Our results show that in adolescence, not only is certain gene activity higher in brain areas crucial for emotional regulation and decision-making, but that nicotine affects key plasticity molecules in these areas in a manner different from that the adult brain. Thus, adolescence may represent a neurobiologically vulnerable period for drug abuse. |
Acknowledgments—This work was supported by National Institute of Heath grant 5 P50 CA084724-04 and National Institute on Drug Abuse grants DA13780 (C.F.L.) and DA14464 (A.E.K.). T.L.S. was supported by National Institute on Drug Abuse Predoctoral National Research Service Award DA00003. Additional support was also provided by the TERN Network of the Robert Wood Johnson Foundation and National Institute on Mental Health grant 1 R24 MH 67346-01. |
|
REFERENCES Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003a;28:1935–1949. [PubMed]Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003b;988:164–172. [PubMed]Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–878. [PubMed]Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. [PubMed]Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. [PubMed]Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl). 2004;174:389–395. [PubMed]Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. [PubMed]Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. [PubMed]Clarke PB, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–708. [PubMed]Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res. 2004a;153:175–187. Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004b;502:75–85. [PubMed]Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. [PubMed]Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. [PubMed]Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. [PubMed]Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. [PubMed]Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. [PubMed]Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. [PubMed]Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav. 2003;74:917–931. [PubMed]Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. [PubMed]Giedd J. Brain development, IX: human brain growth. Am J Psychiatry. 1999;156:4. [PubMed]Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study [letter]. Nat Neurosci. 1999;2:861–863. [PubMed]Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996a;6:551–560. [PubMed]Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996b;366:223–230. [PubMed]Gorren AC, Mayer B. Tetrahydrobiopterin in nitric oxide synthesis: a novel biological role for pteridines. Curr Drug Metab. 2002;3:133–157. [PubMed]Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. [PubMed]Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. [PubMed]Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. [PubMed]Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl). 1999;146:373–390. [PubMed]Kaufman S, Holtzman NA, Milstien S, Butler LJ, Krumholz A. Phenylketonuria due to a deficiency of dihydropteridine reductase. N Engl J Med. 1975;293:785–790. [PubMed]Kellogg CK, Awatramani GB, Piekut DT. Adolescent development alters stressor-induced Fos immunoreactivity in rat brain. Neuroscience. 1998;83:681–689. [PubMed]Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–6451. [PubMed]Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, Tkatch T, Baranauskas G, Surmeier DJ, Neve RL, Duman RS, Picciotto MR, Nestler EJ. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. [PubMed]Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine D1 receptor and is dependent on NMDA stimulation. Brain Res Mol Brain Res. 1994;23:1–13. [PubMed]Kolb B, Nonneman AJ. Functional development of prefrontal cortex in rats continues into adolescence. Science. 1976;193:335–336. [PubMed]Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16:4231–4239. [PubMed]Landry CF, Ivy GO, Dunn RJ, Marks A, Brown IR. Expression of the gene encoding the beta-subunit of S-100 protein in the developing rat brain analyzed by in situ hybridization. Brain Res Mol Brain Res. 1989;6:251–262. [PubMed]Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–159. [PubMed]Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl). 2003;169:141–149. [PubMed]Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. [PubMed]Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. [PubMed]Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. [PubMed]McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. [PubMed]Nauta WJ. The problem of the frontal lobe: a reinterpretation. J Psychiatr Res. 1971;8:167–187. [PubMed]Nisell M, Nomikos GG, Chergui K, Grillner P, Svensson TH. Chronic nicotine enhances basal and nicotine-induced Fos immunoreactivity preferentially in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 1997;17:151–161. [PubMed]O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–174. [PubMed]Ostrander MM, Badiani A, Day HE, Norton CS, Watson SJ, Akil H, Robinson TE. Environmental context and drug history modulate amphetamine-induced c-fos mRNA expression in the basal ganglia, central extended amygdala, and associated limbic fore-brain. Neuroscience. 2003;120:551–571. [PubMed]Pagliusi SR, Tessari M, DeVevey S, Chiamulera C, Pich EM. The reinforcing properties of nicotine are associated with a specific patterning of c-fos expression in the rat brain. Eur J Neurosci. 1996;8:2247–2256. [PubMed]Pandey SC, Roy A, Xu T, Mittal N. Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem. 2001;77:943–952. [PubMed]Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. [PubMed]Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed]Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–86. [PubMed]Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. [PubMed]Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. [PubMed]Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. [PubMed]Salminen O, Seppa T, Gaddnas H, Ahtee L. The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci. 1999;19:8145–8151. [PubMed]Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21:1703–1711. [PubMed]Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psycho-pharmacology (Berl). 2004;175:265–273. Schochet TL, Kelley AE, Landry CF. Differential gene expression in adult and adolescent prefrontal cortex. Society for Neuroscience. 2002 Abstract No. 589.13. Seeman P. Images in neuroscience. Brain development, X: pruning during development. Am J Psychiatry. 1999;156:168. [PubMed]Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. [PubMed]Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. [PubMed]Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. [PubMed]Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. [PubMed]Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. [PubMed]Svenningsson P, Nomikos GG, Fredholm BB. Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci. 1995;15:7612–7624. [PubMed]Tepper JM, Trent F. In vivo studies of the postnatal development of rat neostriatal neurons. Prog Brain Res. 1993;99:35–50. [PubMed]Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. [PubMed]Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. [PubMed]Turner AJ, Ponzio F, Algeri S. Dihydropteridine reductase in rat brain: regional distribution and the effect of catecholamine-depleting drugs. Brain Res. 1974;70:553–558. [PubMed]Vastola B, Douglas L, Varlinskaya E, Spear L. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107. [PubMed]Wang H, Pickel VM. Activity-regulated cytoskeleton-associated protein Arc is targeted to dendrites and coexpressed with muopioid receptors in postnatal rat caudate-putamen nucleus. J Neurosci Res. 2004;77:323–333. [PubMed]Werme M, Olson L, Brene S. NGFI-B and nor1 mRNAs are upregulated in brain reward pathways by drugs of abuse: different effects in Fischer and Lewis rats. Brain Res Mol Brain Res. 2000a;76:18–24. [PubMed]Werme M, Ringholm A, Olson L, Brene S. Differential patterns of induction of NGFI-B, Nor1 and c-fos mRNAs in striatal subregions by haloperidol and clozapine. Brain Res. 2000b;863:112–119. [PubMed]Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends Pharmacol Sci. 1990;11:216–219. [PubMed]Yamagata K, Suzuki K, Sugiura H, Kawashima N, Okuyama S. Activation of an effector immediate-early gene arc by methamphetamine. Ann N Y Acad Sci. 2000;914:22–32. [PubMed]Zetterstrom RH, Williams R, Perlmann T, Olson L. Cellular expression of the immediate early transcription factors Nurr1 and NGFI-B suggests a gene regulatory role in several brain regions including the nigrostriatal dopamine system. Brain Res Mol Brain Res. 1996;41:111–120. [PubMed]Schroeder BE, Binzak JM, Kelley AE. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience. 2001;105:535–545. [PubMed]
|
| |


 The publisher's final edited version of this article is available at
The publisher's final edited version of this article is available at