 |
|
 |

More Individualized Treatment Pursued in Breast Cancer Trial
The launch today of a new clinical trial is being hailed by some of the nation's leading breast cancer researchers as an important step toward more individualized treatment of cancer based on factors such as the expression of specific genes within patients' tumor cells.
The NCI-sponsored trial, dubbed TAILORx, will use the results of a new test that measures the activity of 21 genes in tumor samples from women with early-stage invasive breast cancer that is estrogen-receptor positive and lymph node-negative to assign participants to their treatment regimen.
Read more 1 


New Focus on Lung Cancer Research
Lung cancer continues to be one of the biggest public health challenges facing the United States and many other countries. Although incidence rates have stabilized, more than 173,000 new cases of lung cancer will be diagnosed this year, and it will continue to be the most common cause of cancer death among men and women, with more than 163,000 people succumbing to the disease each year.
NCI recognizes the public health imperative of ensuring that we are doing the most with available resources to tackle this formidable challenge. This recognition led to the formation in 2004 of the Lung Cancer Integration and Implementation (I2) Team 2, one of a handful of special groups including NCI staff and extramural researchers focused on high-priority areas of research. In a Director's Update 3 last summer, Dr. Andrew von Eschenbach praised the Lung Cancer I2 team for their work in identifying gaps and opportunities to accelerate our efforts against lung cancer. I am continuing to lead this effort by creating a base of scientific cohesiveness within the Institute around lung cancer, and will host regular meetings with extramural lung cancer researchers and advocates about our progress in this area and our future agenda. The original I2 team will continue to participate in this effort via monthly conference calls.
Read more 4 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

More Individualized Treatment Pursued in Breast Cancer Trial
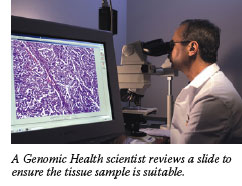 The launch today of a new clinical trial is being hailed by some of the nation's leading breast cancer researchers as an important step toward more individualized treatment of cancer based on factors such as the expression of specific genes within patients' tumor cells.
The launch today of a new clinical trial is being hailed by some of the nation's leading breast cancer researchers as an important step toward more individualized treatment of cancer based on factors such as the expression of specific genes within patients' tumor cells.
The NCI-sponsored trial, dubbed TAILORx, will use the results of a new test that measures the activity of 21 genes in tumor samples from women with early-stage invasive breast cancer that is estrogen-receptor positive and lymph node-negative to assign participants to their treatment regimen.
The Eastern Cooperative Oncology Group (ECOG) is leading TAILORx, and all NCI-sponsored clinical trials groups that perform breast cancer research are participating in the trial.
A number of studies, including one released early online today by the Journal of Clinical Oncology (JCO), have demonstrated that the test - Oncotype DX, developed by Genomic Health, Inc. - is highly accurate at predicting the risk of recurrence among women with this type of cancer and whether they will benefit from adjuvant chemotherapy.
Determining whether to use adjuvant chemotherapy is a critical issue, says Dr. Jo Anne Zujewski, of NCI's Cancer Therapy Evaluation Program. Although about 90 percent of women with this type of breast cancer are advised to undergo adjuvant chemotherapy, she explains, studies have shown that it decreases recurrence risk in only a small percentage of them.
"A large number of these women are receiving toxic chemotherapy unnecessarily, and we need a means of identifying them," Dr. Zujewski says. "TAILORx could help change the way we treat breast cancer, helping to better identify women who are likely to benefit from chemotherapy and those who are not."
In the JCO study, women who had a high "recurrence score" on Oncotype DX - 31 or higher on a 0 to 100 scale - were far less likely to experience disease recurrence over the next 15 years after chemotherapy. For women with low scores (18 or less), adjuvant chemotherapy appeared to have little impact on their recurrence risk. The study, the initial results of which were presented 5 in December 2004, tested tumor samples from 651 patients who received adjuvant tamoxifen and chemotherapy as part of the National Surgical Breast and Bowel Project-conducted B-20 trial.
Although TAILORx should help to confirm these findings, the trial's primary goal, says Dr. Sheila E. Taube, director of NCI's Cancer Diagnosis Program, is to determine whether Oncotype DX can guide the course of treatment for women who have recurrence scores of 11 to 25. The limited number of tumor samples in studies to date that have scored in this range precludes researchers from answering this question. But in TAILORx, nearly 4,400 of the more than 10,000 expected participants will fall into the 11 to 25 range.
In TAILORx, participants with a recurrence score of more than 25 will receive chemotherapy plus hormonal therapy; women with a recurrence score of less than 11 will receive hormonal therapy alone; and women with a recurrence score of 11 to 25 will be randomly assigned to receive adjuvant hormonal therapy, with or without chemotherapy.
By Carmen Phillips
|
 |
 |

New Focus on Lung Cancer Research
Lung cancer continues to be one of the biggest public health challenges facing the United States and many other countries. Although incidence rates have stabilized, more than 173,000 new cases of lung cancer will be diagnosed this year, and it will continue to be the most common cause of cancer death among men and women, with more than 163,000 people succumbing to the disease each year.
NCI recognizes the public health imperative of ensuring that we are doing the most with available resources to tackle this formidable challenge. This recognition led to the formation in 2004 of the Lung Cancer Integration and Implementation (I2) Team 2, one of a handful of special groups including NCI staff and extramural researchers focused on high-priority areas of research. In a Director's Update 3 last summer, Dr. Andrew von Eschenbach praised the Lung Cancer I2 team for their work in identifying gaps and opportunities to accelerate our efforts against lung cancer. I am continuing to lead this effort by creating a base of scientific cohesiveness within the Institute around lung cancer, and will host regular meetings with extramural lung cancer researchers and advocates about our progress in this area and our future agenda. The original I2 team will continue to participate in this effort via monthly conference calls.
We are now launching the NCI Lung Cancer Program (LCP). Using funds from the NCI Director's discretionary budget reserve, the central focus of this program will be to support research into early detection and treatment, efforts we believe are most likely to provide more immediate benefits for lung cancer patients.
An important aspect of the LCP will be initiation of two clinical trials. The first will be a national clinical trial with the primary goal of defining a panel of genomic and proteomic pharmacodynamic markers that can be used to predict response to EGFR inhibitors, such as erlotinib (Tarceva), in patients with non-small-cell lung cancer (NSCLC). The trial will be designed to screen about 1,000 NSCLC patients - enough to identify those with molecular signatures that correlate with erlotinib effectiveness. We hope that this trial will provide a generalized approach for patient selection that could be used as a basis to direct similar investigations.
The second study will begin as an early-phase trial at the NIH Clinical Center to test a DNA methylase inhibitor that has shown the ability to, in effect, reactivate tumor suppressor genes. As a study published earlier this year 6 demonstrated, these genes can be "silenced" when overmethylated, leading to aggressive cellular behavior. This pilot trial offers an opportunity to bring forward a potentially important lung cancer therapeutic agent.
Other parts of the LCP program will include an RFA from the Division of Cancer Biology that will be directed at the biology of very early changes in the lung, inflammation, and the tumor microenvironment. Also, additional resources have been committed to the Cancer Intervention and Surveillance Modeling Network to improve our understanding of the impact of cancer control interventions - specifically in tobacco cessation, early detection and screening, and therapy. Finally, additional funds have been committed to support tissue acquisition, processing, and archiving in the National Lung Screening Trial 7.
While the LCP will not initially increase support to tobacco control programs, NCI will continue to work toward finding resources for those initiatives identified by the I2 Team. Be assured, however, that existing NCI tobacco control research remains a priority, for we cannot forget that the great majority of lung cancers can be prevented through prevention and cessation of smoking and other forms of tobacco use.
Dr. John E. Niederhuber
NCI Deputy Director and Deputy Director for Translational and Clinical Sciences
|
 |
 |

Behind TAILORx, a Push for Clinical Tests
The TAILORx clinical trial, which will evaluate a diagnostic test for breast cancer, is the first trial to be launched under an NCI program designed to bring new clinical tests for common cancers into routine use.
Since it began in 2000, the Program for the Assessment of Clinical Cancer Tests (PACCT) has provided guidance for developing and evaluating biological markers for a variety of purposes, such as diagnosing cancer, assessing prognosis, and predicting a patient's response to therapy.
When the program started, genetic tools such as DNA microarrays were changing the field of cancer diagnostics. Suddenly, it was possible to rapidly identify genes associated with cancer, including potential biological markers for clinical tests.
But despite the tools, remarkably few clinical tests were available to help physicians make treatment decisions for cancer patients. To speed the development of more tests, Dr. Sheila E. Taube, director of NCI's Cancer Diagnosis Program, created PACCT.
"We started PACCT to figure out why there were so few tests being used in the clinic when the literature contained so many reports about promising biological markers," says Dr. Taube.
As a first step, a working group identified some of the barriers to developing tests. These included a lack of regulatory guidance and no standardization in the techniques for developing markers, which made it difficult to compare results across studies.
Next, the team mapped out the steps for identifying and validating biological markers and translating the research into clinically useful tests.
"We determined what the ideal process should be for bringing potential markers through to clinical tests," says Dr. Taube. The process is an iterative loop, she says, in which knowledge gained about an experimental test in the clinic helps refine the test.
The approach stresses the importance of starting with a pressing clinical question that needs to be answered. Once the need is identified, promising markers can be evaluated within that context.
Success depends on having appropriate biological samples for developing markers. For instance, tumor tissue from completed clinical trials can be used to identify markers, but the markers need to be validated in prospective clinical trials, the working group said.
Dr. Taube's team decided to test the strategy by addressing clinical needs in early-stage breast cancer. For some breast cancer patients and their physicians, the most pressing need was for a test that could identify women who needed chemotherapy after surgery.
Studies had suggested that for some women the risk of recurrence was so low that they were unlikely to benefit from the addition of chemotherapy to hormonal therapy after surgery. These women might be spared unnecessary treatment if they could be identified.
In 2002, a PACCT working group was evaluating potential biological markers when Dr. Taube learned about a test being developed by a company called Genomic Health, Inc. The test, Oncotype DX, was designed to predict the risk of breast cancer recurring in certain patients.
"Genomic Health came to us and said they had the answer to our prayers," recalls Dr. Taube. "It turned out that the company had essentially independently followed all of the rules we had set for the ideal way of developing the test."
With support from PACCT, Genomic Health used specimens from previous clinical trials to develop the test in collaboration with NCI Clinical Cooperative Groups. The results, announced in 2004, suggested that the test is useful for identifying women whose risk of recurrence is relatively high or relatively low.
But questions remained about how to treat women whose test scores fell in the middle range. To find answers, PACCT and their collaborators developed the TAILORx trial to determine whether the test can be helpful in selecting the most appropriate treatments for these women.
The results of TAILORx are not expected for a decade, but Dr. Taube believes that Oncotype DX can serve as a model for developing other types of clinical tests for cancer.
By Edward R. Winstead
|
 |
 |
 |
 |
Correction |
 |
|
In the article 8 on esophageal cancer in the May 16 NCI Cancer Bulletin, there was an error in the HTML and PDF versions. Regarding research being conducted
by Dr. Brian Reid and colleagues at Fred Hutchinson Cancer Research Center, the article stated that the researchers were trying to determine whether cell cycle abnormalities were predictive
of progression to esophageal adenocarcinoma in people with Barrett's esophagus. It should have stated "abnormalities in the amount of DNA in a cell," not the "cell cycle." It was corrected on May 17. |
|
Use of Recombinant Human Erythropoietins May Pose Risks
Administration of the drugs epoetin or darbepoetin - recombinant human erythropoietins that stimulate the bone marrow to make red blood cells - can reduce the need for blood transfusions during cytotoxic chemotherapy. However, recent hearings before the Food and Drug Administration questioned whether there is an association between the use of erythropoietins and increased risk of thrombo-embolic events such as stroke. In addition, several papers have presented conflicting evidence on the association between erythropoietins and survival. An updated systematic Cochrane Review published in the May 17 Journal of the National Cancer Institute (JNCI) analyzed data from 57 trials comprising 9,353 patients to better clarify possible risks posed by the use of erythropoietins.
The relative risk of a thrombo-embolic event was found to be significantly increased in patients receiving either epoetin or darbepoetin. In addition, the updated analysis "found no association between treatment and survival and possibly even that decreased survival might be associated with treatment." This result differed from the authors' original review published in 2005, which showed a possible association of erythropoietins with increased survival.
The investigators propose that this difference might be due to newer trials that enrolled nonanemic patients or that aimed to increase hemoglobin to levels higher that those recommended by the product labels, but they did not have the individual patient data needed to clarify these possible associations. Therefore, they advise caution when using either erythropoietin "in combination with chemotherapeutic agents that are known to be thrombogenic or for cancer patients who are at high risk for thrombo-embolic events."
Most Statin Use Not Associated with Breast Cancer Risk
The use of the statins class of cholesterol-lowering drugs was not found to increase the risk of breast cancer, according to results of the Women's Health Initiative (WHI) study reported in the May 17 JNCI.
Previous clinical studies yielded "mixed results" on the association of statins with breast cancer risk. The WHI study of more than 156,000 postmenopausal women collected data on use of statins and other lipid-lowering drugs. Statins were used by 11,710 (7.5 percent) of the women. After an average follow-up of 6.7 years, 4,383 invasive breast cancers were found. Breast cancer incidence was 4.09 per 1,000 person-years among statin users, compared with 4.28 per 1,000 person-years for non-users, the researchers report. The lower incidence among statin users was not statistically significant.
"Overall statin use was not associated with invasive cancer incidence," the researchers note. However, the use of "hydrophobic" statins such as simvastatin, lovastatin, and fluvastatin among 8,106 women was "associated with an 18 percent lower breast cancer incidence," they add. "Our finding that use of hydrophobic statins may be associated with lower breast cancer incidence suggests possible within-class differences that warrant further evaluation."
This observation "is consistent with a cell culture study in which only hydrophobic statins (lovastatin, simvastatin, and fluvastatin) but not a hydrophilic statin (pravastatin) had anticancer activity," the researchers explain. "Our results, taken together with the existing literature, indicate that breast cancer risk is at least not increased in statin users. Whether or not statin use is associated with reduced breast cancer risk is less certain."
Combination of Antibody-Based Therapies Kills Tumors in Mice
Researchers have used a combination of antibody-based therapies to eradicate established tumors in mice. The combination included three monoclonal antibodies that together caused the death of tumor cells and the activation of tumor-specific immune cells.
A team from the Juntendo University School of Medicine in Tokyo induced tumor cell death, or apoptosis, using a monoclonal antibody that acts through a receptor called DR5; they activated tumor-specific CD8-positive T cells using monoclonal antibodies against the molecules CD40 and CD137. The combination, which the researchers call trimAb, "potently" induced the rejection of established tumors in most mice tested.
TrimAb therapy eradicated mammary tumors in 80 percent of mice tested, whereas other combined or single monoclonal antibody treatments did so in less than 30 percent of mice tested. When the researchers treated larger tumors, trimAb therapy eradicated tumors in 70 percent of mice, whereas the rate was less than 20 percent for all other combinations, according to findings published online in Nature Medicine. The researchers also tested tumors of the colon and the lung.
No side effects of trimAb therapy were observed in the mice, and the researchers say it would be important to minimize autoimmune reactions in adapting the therapy for humans. More research is needed, but the current results suggest that a combination therapy that both causes tumor-cell apoptosis through DR5 and activates T cells "may be an effective strategy for cancer immunotherapy in humans," the researchers conclude.
|
 |
 |

 |
 |
CCR Grand Rounds |
 |
|
May 30: Dr. Allan Balmain, Barbara Bass Bakar Distinguished Professor of Cancer Genetics, Department of Biochemistry and Biophysics, UCSF Comprehensive Cancer Center, University of California, San Francisco. "Mouse Models for the Identification of Human Tumor Susceptibility Genes."
June 6: No lecture. ASCO Annual Meeting, June 2-6, Atlanta.
CCR Grand Rounds are held 8:30 to 9:30 a.m. at the NIH campus in Bethesda, Md., in the Clinical Center's Lipsett Amphitheater. |
|
In May, the Food and Drug Administration (FDA) took action on two drugs related to cancer.
Committee Recommends HPV Vaccine Approval
An advisory committee to the FDA last week unanimously recommended that the agency approve a vaccine that protects against four types of human papillomavirus (HPV). The vaccine, Gardasil, manufactured by Merck, has shown 100 percent efficacy in preventing infection with the two HPV types that cause 70 percent of cervical cancer cases, HPV 16 and 18.
The agency's Vaccines and Related Biological Products Advisory Committee unanimously agreed that data from phase II and III clinical trials conducted in the United States, Europe, and South America - which included more than 20,000 participants - supported that the vaccine is safe and effective in preventing HPV-related cervical cancer as well as precancerous cervical, vaginal, and vulvular lesions.
The committee's chair, Dr. Monica Farley, stressed that, if the FDA approves the vaccine, postmarket studies of Gardasil to ensure its long-term effectiveness and safety would be very important.
Merck submitted a Biologics License Application to the FDA for the marketing approval of Gardasil last December, and the agency has said it will make a decision on the application by June 8. Gardasil and another HPV vaccine manufactured by GlaxoSmithKline were developed on the basis of work originally
done 9 by NCI investigators.
Although Merck has only conducted clinical trials to test the vaccine's effectiveness in women 16-26 years old, it requested approval for use in females ages 9-26. Logistical and ethical concerns have precluded the company from conducting efficacy trials in girls 9-15, so the company instead conducted immunogenicity trials in girls of this age to measure their immune response to the vaccine.
Younger females had an even stronger immune response than the older females in the efficacy trials, explained Dr. Eliav Barr of Merck. The advisory committee unanimously agreed that these data supported "bridging" or extending the effectiveness data to include this younger group.
Several committee members commented that, if Gardasil does come to market, it will be very important to stress it's not a substitute for cervical cancer screening.
New Smoking Cessation Medication Approved
On May 11, the FDA approved Chantix (varenicline tartrate), a new medication made by Pfizer to help cigarette smokers quit. Chantix acts on the brain's nicotine receptors and may help people quit by providing some of the nicotine effects to ease withdrawal symptoms and by blocking the effects of nicotine if the person resumes smoking.
Chantix is approved for use in adults and is taken for 12 weeks; patients who successfully quit may take an additional 12 weeks of the medication to increase their likelihood of long-term cessation. The data that led to the approval of Chantix came from 6 clinical trials including more than 3,600 patients; 5 of the 6 studies were randomized controlled clinical trials in which Chantix was shown to be superior to placebo. All the trials provided patients with educational materials on smoking cessation and up to 10 minutes of counseling at each weekly treatment visit.
About 1 in every 5 adult Americans (44.5 million) smokes cigarettes, and each year, more than 440,000 Americans die prematurely from illnesses caused by tobacco use. "FDA's approval of Chantix provides an additional option for people who smoke to quit," said Dr. Glen Morgan of NCI's Tobacco Control Research Branch. "NCI recommends that smokers employ behavioral techniques with every quit attempt." Information and assistance with smoking cessation can be found at www.smokefree.gov or by calling
1-800-QUIT-NOW. |
 |
 |

Novel Technologies for In Vivo Imaging
Announcement Number: PA-06-398
New Application Receipt Dates: June 1 and Oct. 1, 2006.
This is a renewal of PA-04-095 and will use the R21 and R33 award mechanisms. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3456. Inquiries: Dr. Guoying Liu - guoyingl@mail.nih.gov; Dr. Keyvan Farahani - farahank@mail.nih.gov; Dr. James A. Deye - deyej@mail.nih.gov; Dr. Houston Baker - bakerhou@mail.nih.gov
Novel Technologies for In Vivo Imaging
Announcement Number: PA-06-399
New Application Receipt Dates: June 1 and Oct. 1, 2006.
This is a renewal of PA-04-095 and will use the R33 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3457. Inquiries: Dr. Guoying Liu - guoyingl@mail.nih.gov; Dr. Keyvan Farahani - farahank@mail.nih.gov; Dr. James A. Deye - deyej@mail.nih.gov; Dr. Houston Baker - bakerhou@mail.nih.gov
Developmental Projects in Complementary Approaches to Cancer Care
Announcement Number: PA-06-400
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1, 2009.
This is a renewal of PA-04-053 and will use the R21 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3458. Inquiries: Dr. Wendy B. Smith - smithwe@mail.nih.gov
Studies of Energy Balance and Cancer in Humans
Announcement Number: PA-06-404
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1, 2009.
This is a renewal of PA-04-124 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3459. Inquiries: Dr. Virginia W. Hartmuller - hartmulv@mail.nih.gov; Dr. Noreen M. Aziz - na45f@nih.gov; Dr. Sharon A. Ross - rosssha@mail.nih.gov
In Vivo Cellular and Molecular Imaging Centers
Announcement Number: PAR-06-406
Letter of Intent Receipt Dates: July 16, 2006; July 16, 2007. Application Receipt Dates: Aug. 16, 2006; Aug. 16, 2007.
This is a renewal of PAR-04-069 and will use the P50 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3462. Inquiries: Dr. Anne E. Menkens - menkensa@mail.nih.gov
New Technologies for Liver Disease SBIR
Announcement Number: PA-06-397
Non-AIDS Application Receipt Dates: Aug. 1 and Dec. 1, 2006; April 1, Aug. 1, and Dec. 1, 2007; April 1, Aug. 1, and Dec. 1, 2008.
This funding opportunity will use the R43 and R44 award mechanisms. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3455. Inquiries: Dr. Asad Umar - au9q@nih.gov; Dr. Phil Daschner - PD93U@nih.gov
New Technologies for Liver Disease STTR
Announcement Number: PA-06-396
Non-AIDS Application Receipt Dates: Aug. 1 and Dec. 1, 2006; April 1, Aug. 1, and Dec. 1, 2007; April 1, Aug. 1, and Dec. 1, 2008.
This funding opportunity will use the R41 and R42 award mechanisms. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3454. Inquiries: Dr. Asad Umar - au9q@nih.gov; Dr. Phil Daschner - PD93U@nih.gov
Global Research Initiative Program, Basic/Biomedical Sciences
Announcement Number: PAR-06-394
Letter of Intent Receipt Dates: Aug. 21, 2006; Aug. 21, 2007; Aug. 21, 2008.
New Application Receipt Dates: Sept. 21, 2006; Sept. 21, 2007; Sept. 21, 2008.
This is a renewal of PAR-03-118 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3461. Inquiries: Dr. Susan A. McCarthy - mccarths@mail.nih.gov
International Tobacco and Health Research and Capacity Building Program
Announcement Number: RFA-TW-06-006
Letter of Intent Receipt Date: Aug. 25, 2006. Application Receipt Date: Sept. 25, 2006.
This is a renewal of RFA-TW-02-005 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3463. Inquiries: Dr. Michele Bloch - blochm@mail.nih.gov
Innovations in Biomedical Computational Science and Technology
Announcement Number: PAR-06-410
Application Receipt Dates: June 24 and Sept. 24, 2006; Jan. 24, May 24, and Sept. 24, 2007; Jan. 24, May 24, and Sept. 24, 2008; Jan. 24, 2009.
This is a renewal of PAR-03-106 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3471. Inquiries: Dr. Peter Lyster - lysterp@mail.nih.gov
Innovations in Biomedical Computational Science and Technology
Announcement Number: PAR-06-411
Application Receipt Dates: June 24 and Sept. 24, 2006; Jan. 24, May 24, and Sept. 24, 2007; Jan. 24, May 24, and Sept. 24, 2008; Jan. 24, 2009.
This is a renewal of PAR-03-106 and will use the R21 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3474. Inquiries: Dr. Peter Lyster - lysterp@mail.nih.gov
Diet, Epigenetic Events, and Cancer Prevention
Announcement Number: PA-06-412
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1 and June 1, 2009.
This is a renewal of PA-04-099 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3466. Inquiries: Dr. Sharon A. Ross - rosssha@mail.nih.gov
Diet, Epigenetic Events, and Cancer Prevention
Announcement Number: PA-06-413
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1 and June 1, 2009.
This is a renewal of PA-04-099 and will use the R21 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3467. Inquiries: Dr. Sharon A. Ross - rosssha@mail.nih.gov
Diet, Epigenetic Events, and Cancer Prevention
Announcement Number: PA-06-414
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1 and June 1, 2009.
This is a renewal of PA-04-099 and will use the R03 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3468. Inquiries: Dr. Sharon A. Ross - rosssha@mail.nih.gov
Exploratory/Developmental Bioengineering Research Grants (EBRG)
Announcement Number: PA-06-418
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1 and June 1, 2009.
This is a renewal of PA-03-058 and will use the R21 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3469. Inquiries: Dr. Houston Baker - bakerhou@mail.nih.gov
Exploratory/Developmental Bioengineering Research Grants (EBRG)
Announcement Number: PA-06-419
New Application Receipt Dates: June 1 and Oct. 1, 2006; Feb. 1, June 1, and Oct. 1, 2007; Feb. 1, June 1, and Oct. 1, 2008; Feb. 1 and June 1, 2009.
This is a renewal of PA-02-011 and will use the R01 award mechanism. For more information, see http://cri.nci.nih.gov/4abst.cfm?initiativeparfa_id=3470. Inquiries: Dr. Houston Baker - bakerhou@mail.nih.gov |
 |
 |

Tailored Treatment for Breast Cancer
Name of the Trial
Phase III Randomized Study of Adjuvant Combination Chemotherapy and Hormonal Therapy Versus Adjuvant Hormonal Therapy Alone in Women with Previously Resected Axillary Node-Negative Breast Cancer with Various Levels of Risk for Recurrence (TAILORx Trial) (ECOG-PACCT-1). See the protocol summary at http://cancer.gov/clinicaltrials/ECOG-PACCT-1.
Principal Investigator
Dr. Joseph A. Sparano, Study Chair, Eastern Cooperative Oncology Group
Why This Trial Is Important
Adding chemotherapy to hormone therapy in the adjuvant (after surgery) treatment of women with breast cancer has been shown to reduce the risk of breast cancer recurrence. However, the additional benefit provided by chemotherapy is small for women whose lymph nodes are free of disease and whose tumors are positive for the estrogen hormone receptor. Because chemotherapy can cause serious side effects, doctors want to find ways to identify patients who will benefit from chemotherapy and those who may be able to avoid it because of little added benefit.
In this trial, doctors will use a test called the Oncotype DX Breast Cancer Assay, which measures the activity of a set of genes in breast tumor tissue. Previous research has shown that a low score in this assay identifies women who will benefit little from chemotherapy, whereas those with a high score benefit substantially. More information is needed about women with scores in the middle range.
Patients will be assigned to one of three major groups. Those with Oncotype DX scores <= 10 will be treated with adjuvant hormone therapy only. Women with scores of 11 to 25 will be randomly assigned to adjuvant hormone therapy alone or adjuvant combination chemotherapy and hormone therapy. Women with scores >= 26 will be treated with adjuvant combination chemotherapy and hormone therapy.
Who Can Join This Trial
Researchers will enroll 10,046 women aged 18 to 75 with ER- and/or PR-positive breast cancer that has not spread to the lymph nodes and can be surgically removed. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/ECOG-PACCT-1.
Study Sites and Contact Information
Study sites in the United States are recruiting patients for this trial. See the list of study contacts at http://cancer.gov/clinicaltrials/ECOG-PACCT-1 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237) for more information. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |
 |
 |
 |
 |
 |
 |
Learn About NCI at ASCO |
 |
|
Be sure to visit the NCI Exhibit in Booth #122 at the American Society for Clinical Oncology (ASCO) annual meeting in Atlanta on June 2-6, to learn about the latest NCI publications, grants and funding opportunities, and training programs. NCI staff from the cancer Biomedical Informatics Grid (caBIG), the Center for Cancer Research, and the Comprehensive Minority Biomedical Branch will be on hand to discuss their programs throughout the meeting. In addition, "Meet the Experts" sessions will take place in the booth according to the schedule below.
Saturday, June 3
10:00-11:00 a.m.: Dr. Julia Rowland, Cancer Survivorship Research Opportunities, Careers, and Training in Psychosocial Oncology
12:00-1:00 p.m.: Dr. Yvonne Vargas, Cancer Prevention Fellowship: Opportunities and Challenges
1:00-2:00 p.m.: Dr. Ernest Hawk, Translational Research Working Group
2:00-3:00 p.m.: Dr. Jonathan Wiest, Interagency Oncology Task Force, Research and Regulatory Review Fellowship Program
Sunday, June 4
10:00-11:00 a.m.: Dr. Ernest Hawk, Translational Research Working Group
Monday, June 5
9:00-10:00 a.m.: Dr. Heng Xie, Grants Process and Funding Support
12:00-1:00 p.m.: Dr. Yvonne Vargas, Cancer Prevention Fellowship: Opportunities and Challenges
1:00-2:00 p.m.: Dr. James L. Gulley, Clinical Vaccine Program
|
 |
|
 Fraumeni Wins IARC Award
Fraumeni Wins IARC Award
On May 17, Dr. Joseph F. Fraumeni, Jr., director of NCI's Division of Cancer Epidemiology and Genetics, was presented with the Medal of Honor from the International Agency for Research on Cancer (IARC) in Lyon, France, in recognition of his "outstanding contributions to the field of cancer epidemiology." He also delivered the third Richard Doll Lecture, entitled "Genes and the Environment in Cancer Causation: An Epidemiologic Perspective."
Previous winners of the IARC Medal of Honor include Dr. Umberto Veronesi of the European Institute of Oncology, Dr. Jeffrey Koplan of the Centers for Disease Control and Prevention, and Professor David Lane of the University of Dundee in the United Kingdom. The inaugural Richard Doll Lecture was given by Sir Richard Doll of Oxford University, and the second by Dr. Brian MacMahon of Harvard University.
Advocacy Conference Registration Deadline is May 31
The registration deadline for "Listening and Learning Together: Building a Bridge of Trust," the summit meeting for advocates sponsored by NCI's Office of Liaison Activities and the NCI Director's Consumer Liaison Group, is May 31. The June 19-20 event at the Natcher Conference Center is free, but all participants must preregister; there will be no onsite registration. Online registration is available at http://www.palladianpartners.com/NCISummit2006/index.htm.
Symposium Presents Health Communication Research
Almost 400 people attended the Symposium on State-of-the-Science Health Communication on May 10, sponsored by NCI's Health Communication and Informatics Research Branch (HCIRB) in the Division of Cancer Control and Population Sciences. The meeting featured insights and research highlights presented by principal investigators from the four NCI-funded Centers of Excellence in Cancer Communication Research. Participants included staff from NCI, several other NIH institutes, the Centers for Disease Control and Prevention, and members of the external research community. Presentations are posted on the HCIRB Web site (http://dccps.nci.nih.gov/hcirb/).
|
|
 |
 |

MECC Tracks Cancer Incidence through International Collaboration
 The Middle East Cancer Consortium (MECC) was established in 1996, when the Ministers of Health of Egypt, Israel, Jordan, Cyprus, and the Palestinian Authority (PA) signed an agreement to work together to reduce the incidence and impact of cancer in the Middle East through collaborative research. Turkey joined the Consortium in 2004. One of the first steps taken by MECC was to set up a Joint Cancer Registration Project, in which a population-based cancer registry would be created (or enhanced if one already existed) in each member state, and data collected on the incidence of cancer. After some intense preparation, the project was launched in January 1998, and was guided and coordinated by a Steering Committee (chaired by myself) comprising members from the participating registries and outside advisors.
The Middle East Cancer Consortium (MECC) was established in 1996, when the Ministers of Health of Egypt, Israel, Jordan, Cyprus, and the Palestinian Authority (PA) signed an agreement to work together to reduce the incidence and impact of cancer in the Middle East through collaborative research. Turkey joined the Consortium in 2004. One of the first steps taken by MECC was to set up a Joint Cancer Registration Project, in which a population-based cancer registry would be created (or enhanced if one already existed) in each member state, and data collected on the incidence of cancer. After some intense preparation, the project was launched in January 1998, and was guided and coordinated by a Steering Committee (chaired by myself) comprising members from the participating registries and outside advisors.
The registries were situated in Nicosia, Cyprus; Tanta, Egypt; Jerusalem, Israel; Gaza City, PA Gaza; Beit Jalla, PA West Bank; and Izmir, Turkey. From the outset, we paid close attention to data comparability and quality, creating a manual of standards for coding information and programs for checking the standardization of the coding and the completeness of registration. Staff at each registry received regular training in cancer registration. These activities were led by Dr. John Young of Emory University in Atlanta. In addition, we adopted the standard computer software known as CANREG, provided and supported by the International Agency for Research on Cancer, for entering and storing the registration data.
The coordination of the project has never been simple and has often been disrupted by political events in the region, but through persistence of purpose, patience, and goodwill, the major aims of the project - to produce an accurate picture of cancer incidence patterns in the region covered by MECC - are being realized. Ten years after its establishment, this year, MECC has published a monograph, Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER. A few of the major findings are:
- Jordanians had the lowest overall incidence of cancer; the U.S. SEER population and Israeli Jews had substantially higher incidence, while Cypriots, Israeli Arabs, and Egyptians had intermediate incidence.
-
Liver cancer incidence rates in Egyptians were five to seven times as high as those of the other MECC populations, and more than three times the U.S. SEER population, possibly related to the higher prevalence of hepatitis B and C in Egypt.
- Egyptians and Israeli Jews had rates of non-Hodgkin lymphoma higher than in the U.S. SEER population and considerably higher than in the other MECC populations. Further studies of risk factors for this malignancy are needed in this region of the world.
- Many more interesting results can be found in the monograph, which is available on the MECC Web site at http://mecc.cancer.gov under Cancer Registry Project.
This project represents a decade of hard work and collaboration between scientists from each of these countries and is a model for international scientific collaboration in troubled regions of the world. It would not have been possible without the leadership of the MECC Executive Director, Prof. Michael Silbermann, the support of NIH and NCI, and the guidance and encouragement of Dr. Joe Harford, Director of NCI's Office of International Affairs, and the primary liaison to MECC.
Dr. Laurence Freedman
Director, Biostatistics Unit
Sheba Medical Center
Tel Hashomer, Israel
|
 |
|
Table of Links
| 1 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_052306/page2 |
| 2 | http://www.cancer.gov/aboutnci/Lung-Cancer-Integration-and-Implementation-Team-
I2 |
| 3 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_081605/page3 |
| 4 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_052306/page3 |
| 5 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_121404/page2 |
| 6 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_013106/page5#b |
| 7 | http://www.cancer.gov/nlst |
| 8 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_051606/page4 |
| 9 | http://www.cancer.gov/directorscorner/directorsupdate-11-30-2004 |
|
 |