News
Finding What's Real, Functionally Speaking
Not all mutations are created equal. Where some can be silent, having no discernable effect on a cell, tissue, or organ, others can be profoundly disruptive.¹ Deciding which category a mutation fits into is often the subject of significant amounts of experimental work and debate, particularly when the gene or genes in question could potentially raise or lower the risks of developing diseases like cancer.
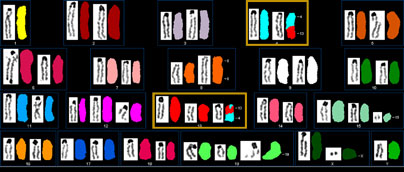
The functional consequences of mutations in BRCA2 (represented here in a multispectral karyotype displaying a translocation between chromosomes 4 and 13) and other genes can now be better and more rapidly understood thanks to a new in vitro validation system based on mouse embryonic stem cells.
Such is the case for the DNA repair genes BRCA1 and BRCA2. Mutations in these genes can significantly increase a woman’s risk of developing breast cancer. While sequencing-based tests can help women assess their risk and make appropriate decisions about preventive treatment, the functional impacts of nearly 1,900 identified BRCA1 and BRCA2 mutations on the genes’ protein products remain unclear. Tying mutations to functions would help improve breast cancer risk assessments; however, such functional studies are both labor- and resource-intensive.
CCR’s Shyam Sharan, Ph.D., Head of the Mouse Genetics Program’s Genetics of Cancer Susceptibility Section, and Research Fellow Sergey Kuznetsov, Ph.D., have developed an in vitro functional assay system capable of accurately and relatively rapidly measuring the effects of BRCA2 mutations. The pair, together with Pentao Liu, Ph.D. (a former CCR Research Fellow now at the Wellcome Sanger Trust Institute), published a report on their system in the July, 2008, issue of Nature Medicine.
The system measures a mutation’s functional impact using a knockout-and- replace strategy. The researchers replaced both normal Brca2 gene copies in mouse embryonic stem cells (ESCs) with copies of human BRCA2 containing particular mutations of interest. Measurements of the cells’ growth, survival, and sensitivity to agents (chemicals, radiation, etc.) that cause DNA damage indicated which mutations had no effect and which kept BRCA2 from compensating for the loss of Brca2.
Using this system, Sharan, Kuznetsov, and Liu examined the functional impacts of 17 catalogued BRCA2 mutations. Their findings validated the known effects of 13 of the mutations and gave the first evidence of the impacts of four others whose functional impacts had not been characterized previously. The researchers noted that with their system, between three and five mutations can be assessed on a two- to three-month timeframe, and the system could potentially be used with any gene mutation that causes a phenotype in mouse ESCs, making this system a useful tool for genetic counselors. They caution, though, that the system needs to be validated further before it is ready for clinical application.