About the Associate Director

Jerry M. Collins, Ph.D., is an internationally recognized pharmacologist. His distinguished career has been closely associated with NCI's drug development efforts for more than 25 years, first as an NCI intramural investigator and then as the chief of the Pharmacokinetics Section.
More...Overview
The Developmental Therapeutics Program (DTP) has played an intimate role in the discovery or development of 40 U.S.-licensed chemotherapeutic agents, with the rest coming directly from the pharmaceutical industry.
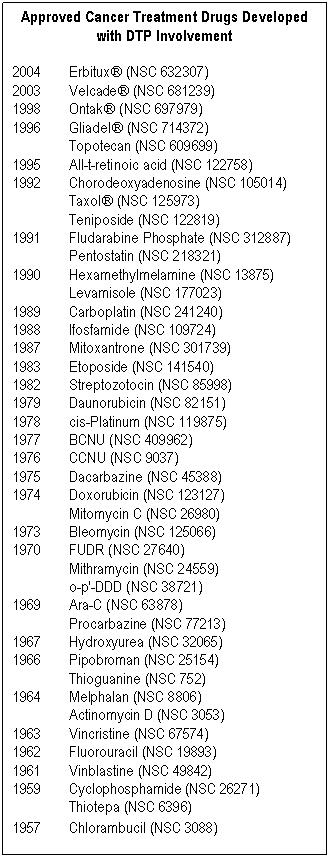 DTP's roster of drug success stories is impressive. On that list is paclitaxel (Taxol®), one of the most widely prescribed anticancer drugs on the market. Paclitaxel, a natural product, was first harvested by researchers working under a joint U.S. Department of Agriculture-National Cancer Institute (NCI) grant. It was a DTP contractor who formulated the drug for use in clinical trials. Bortezomib (Velcade®) is another DTP success story. In cooperation with its commercial sponsor, bortezomib was screened and formulated by DTP. Approved by the FDA in 2003, it remains the first treatment in more than a decade to be approved for patients with multiple myeloma.
DTP's roster of drug success stories is impressive. On that list is paclitaxel (Taxol®), one of the most widely prescribed anticancer drugs on the market. Paclitaxel, a natural product, was first harvested by researchers working under a joint U.S. Department of Agriculture-National Cancer Institute (NCI) grant. It was a DTP contractor who formulated the drug for use in clinical trials. Bortezomib (Velcade®) is another DTP success story. In cooperation with its commercial sponsor, bortezomib was screened and formulated by DTP. Approved by the FDA in 2003, it remains the first treatment in more than a decade to be approved for patients with multiple myeloma.
DTP has been involved in the discovery or development of over 70 percent of the anticancer therapeutics on the market today. Although many academic and private-industry laboratories also are focused on drug discovery, financial and technical burdens as well as lack of funding and infrastructure present barriers that may keep promising therapeutics from reaching patients. DTP helps to overcome therapeutic development barriers by supporting high-risk projects.
In keeping with its goal to turn molecules into medicine for the public health, DTP, created by Congress in 1955 as the Cancer Chemotherapy National Service Center, serves as a vital resource in acquiring preclinical information and providing research materials, including Web-accessible data and tools, vialed and plated compounds, tumor cells, animals, and providing bulk drugs for investigational new drug (IND)-directed studies.
DTP's staff and administered grants are divided among nine components:
- Biological Resources Branch
- Biological Testing Branch
- Drug Synthesis and Chemistry Branch
- Grants and Contracts Operations Branch
- Information Technology Branch
- Natural Products Branch
- Pharmaceutical Resources Branch
- Screening Technology Branch
- Toxicology and Pharmacology Branch
In 2005, DTP administered 581 investigator-initiated, peer-reviewed grants.

