In The Clinic
Ovarian Cancer:
A Silent Killer "Speaks" through Proteins
Targeting the vascular endothelial growth factor (VEGF) pathway, well known to be a critical pathway for the process of angiogenesis is a hypothesis currently being explored in our clinic. Angiogenesis is a normal physiological process that occurs when new blood vessels grow from existing blood vessels. In 1971, the late Judah Folkman, M.D., first proposed that tumors relied on angiogenesis for survival; if they were denied this blood supply, the tumors would die. In 1974, Lance Liotta, M.D., Ph.D., demonstrated that angiogenesis was necessary for metastasis, the process of cancer dissemination. After decades of disregard, angiogenesis became widely accepted throughout the scientific and medical communities, and the field of anti-angiogenesis therapy was born. In the clinical trials being conducted here at CCR, such therapies are used in an attempt to "starve" the ovarian tumors.
My team and I have recently reported on the safety and efficacy of a combination of two agents that block angiogenesis: bevacizumab (Avastin®) and sorafenib (Nexavar®). Although both agents target the VEGF pathway, each does it through different mechanisms (Figure 1). Bevacizumab, FDA-approved for non-small cell lung cancer and metastatic colorectal and breast cancers, is an anti-VEGF monoclonal antibody that prevents VEGF from binding to its receptor (VEGFR). Sorafenib, FDA-approved for advanced renal cell carcinoma and hepatocellular carcinoma, is a small molecule drug that blocks VEGFR2 and downstream signals that are activated by VEGF.
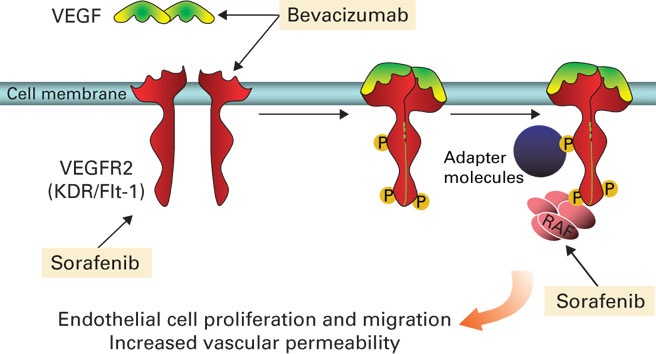
Figure 1: Kohn and colleagues are the fi rst to target the VEGF signaling pathway in series by combining the anti- angiogenesis treatments bevacizumab and sorafenib. Ongoing clinical trials indicate that this approach inhibits the pathway at two different points; as such the combination therapy holds promise for the treatment of refractory or recurrent ovarian cancer.
Our hypothesis is that targeting the VEGF pathway in series rather than in parallel will enhance the effects of both agents. We are also inhibiting the pathway at two different points—in endothelial (blood cells) and epithelial cells (ovarian tumor cells)—using this strategy. Our clinic is the first to target VEGF signalingin series with combination specific antiangiogenesis therapy.
There are two clinical trials under investigation using this combined treatment. In a Phase I study, 62 patients with refractory, metastatic, or unresectable solid tumors of any type have been enrolled. This study is addressing identification of optimal doses, safety, and toxicity of this regimen in these patient populations. Tumor samples have been obtained from which to measure changes in the targeted protein networks and correlate them to a clinical outcome.
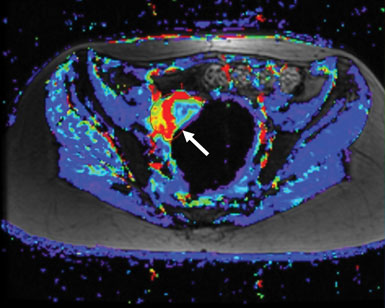
The second study using this combination therapy is a Phase II study specifically for patients with recurrent ovarian, fallopian tube, or primary peritoneal cancers. The objective of this trial is to confirm potential benefit of sorafenib and bevacizumab in these patients and to help guide further application of the regimen outside of NCI. Initial Phase I data in these patient populations showed promising activity in tumors known to have increased VEGF pathway signaling, but with synergistic anti-tumor activity at doses below the standard single agent treatment doses. Thirty-three percent of all treated patients had some reduction in tumor size—some quite rapidly—and many of the rest saw their tumors stabilize (Figure 2). Combination therapy reduced the blood supply to many patients' tumors. We observed a greater benefit than was expected in a Phase I clinical trial, and this has given us hope that these results will be reproduced in the ovarian cancer Phase II study.
We observed a greater benefit than was expected in a Phase I clinical trial, and this has given us hope that these results will be reproduced in the ovarian cancer Phase II study.
We will analyze patient tumor samples, collected prior to treatment and while patients were on therapy, to investigate whether those who had a good response to treatment displayed an initially overactive VEGF pathway or one inhibited by treatment. The resulting data could provide further justification for tailoring therapy to a tumor's protein profile and could result in a companion predictive test for this combination therapy, allowing doctors to monitor response during treatment.
Diagnostic Biomarkers
The lack of a validated screening test for ovarian cancer has prompted investigators to seek alternative diagnostic strategies. Tumors leak proteins into body fluids, including blood and urine, and some of these proteins may be able to alert doctors to the presence of disease. These cancer-related proteins are known as cancer biomarkers. By collecting these fluids, it may be possible to develop a biomarker that may diagnose cancer at an early stage.
Biomarker use is not a new concept. Elevated prostate specific antigen (PSA) is an example of a biomarker that can be detected in men who have organlimited prostate cancer. Technologies for detecting proteins and our understanding of the underlying relationship between proteins and cancer have come a long way. These scientific advancements are being translated to clinical trials to benefit our patients.
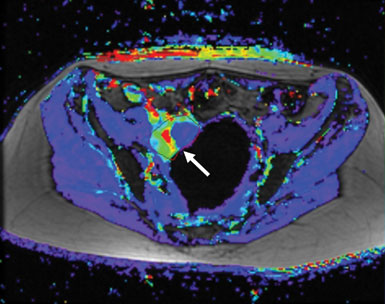
Figure 2: The difference in a tumor’s blood flow before (left, red and yellow region next to arrow) and after (right) treatment with a combination of anti-angiogenic therapies can be striking. In a Phase II clinical trial, thirty-three percent of patients treated with sorafenib and bevucizumab, which target different components of the angiogenic pathway, saw some reduction in tumor size.
My ovarian cancer team and I are in collaborations to analyze blood samples from ovarian cancer patients for protein "signatures," or patterns of proteins, that can predict early-stage ovarian cancer and cancer recurrence. In particular, candidate biomarkers will be compared against or tested alongside the CA-125 biomarker to determine whether they are more effective than this biomarker in predicting ovarian cancer's return. In order to carry out this biomarker research, my colleagues and I are developing a repository, or bank, of blood samples from patients enrolled in one of the clinical trials. Because few, if any, cancers are characterized by a single reliable biomarker, like PSA, this sample collection is critical. We must collect and analyze a large number of blood samples. This trial is designed to accrue samples from 400 women in order to have confidence that the initual protein signatures may have true value in predicting ovarian cancer relapse. We remain faced with the challenge of determining which biomarkers will, when combined, form the most sensitive, specific, and reliable test for disease.
2 Liotta LA, Kohn EC, Petricoin EF. Clinical proteomics, personalized molecular medicine. JAMA. 2001;286(18):2211-2214. To learn more about Dr. Kohn's work, visit http://ccr.cancer.gov/staff/staff.asp?profileid=5844


