Features
The DNA of Drug Discovery
Drug discovery, like research in general, relies on a fine balance between directed exploration and serendipity. With the goal of translating basic scientific insights into cures, CCR fosters this balance by providing the infrastructure to make new connections among seemingly disparate research efforts—both within the NCI and extramurally and by providing new tools and opportunities for investigators to follow the therapeutic directions generated by their science.
Yves Pommier, M.D., Ph.D., Chief of CCR's Laboratory of Molecular Pharmacology, has invested his career in studying DNA processing mechanisms, with an eye towards turning his mechanistic insights into new generations of drugs. And thanks to innovative collaborations within and beyond NCI that have bridged his knowledge of molecular biology with the expertise of chemists, such drugs may be closer to hand.
Camptothecins: From Tree Bark to Topoisomerase
To fully understand the story behind Pommier's quest, one must look back 40 years. In the 1960s, while working on a contract with NCI, Monroe Wall, Ph.D., whose credits already included the purification of the anti-cancer wonder drug paclitaxel (Taxol®) from the bark of the Pacific yew tree, identified a second cancer-fighting compound— camptothecin—from the bark of a tupelo tree found only in China and Tibet. Wall studied camptothecin and synthesized derivatives, but without a known mechanism of action, the compounds languished at NCI's Natural Products Branch. Some 20 years later, in 1985, an NCI-supported academic/commercial collaboration of researchers at Johns Hopkins University, University of Florida, and SmithKline (now GlaxoSmithKline, or GSK) provided the first evidence that a DNA processing enzyme called topoisomerase I (topo I)—which makes cuts in DNA double helices, permitting them to relax for transcription or replication—was the camptothecins' molecular target.
...If there is one family of topo I inhibitors, might not there be another that would prove more powerful still?
At the time the camptothecinstopo I link was announced, Pommier's group was studying topoisomerase II (topo II), a related enzyme and known target of chemotherapeutic agents like doxorubicin. Thus, he was well positioned to study the cellular mechanisms of action of this new class of compounds. He and others confirmed that topo I was indeed the camptothecins' anti-cancer target and that the drugs turned normal topo I into a deadly enzyme by jamming it irreversibly onto the cell's DNA. Pommier's group also showed that human cancer cell lines could evolve resistance to camptothecins, invariably due to a mutation in the topo I gene. Within ten years of the confirmation of topo I's role, two camptothecin drugs had been FDA approved—topotecan (Hycamtin®) and irinotecan (Camptosar®).
Limited by their chemical stability and toxicity, camptothecins were not suitable for widespread drug development efforts. However, if there is one family of topo I inhibitors, might not there be another that would prove more powerful still? This was the question that Pommier and his colleagues decided to attack. But to do so, they needed help.
Panning for New Topo I Inhibitors
Prior to his death a few years ago, Ken Paull, Ph.D., worked with NCI's Developmental Therapeutics Program (DTP) to screen compounds for anti-cancer activities. NCI has 60 distinct standardized cancer cell lines, the so-called NCI-60, that its scientists use to screen compounds at five different concentrations for their ability to inhibit growth. Since no two cell lines are identical, compounds with different mechanisms of action affect the proliferation of individual cancer cell lines differently. Paull and his colleagues realized that by comparing dose-response profiles across all 60 cell lines, they could classify compounds with related mechanisms of action; drugs that affect all of the cell lines in a similar way are likely to operate via a similar mechanism. Paull formalized this logic in a computer algorithm called COMPARE.
Familiar with Paull's work, Pommier decided to see if COMPARE could pick out compounds that work like camptothecin. When they struck gold, the compound they identified, an indenoisoquinoline, turned out to be the byproduct of another serendipitous event captured by the NCI. The compound, synthesized by chemist Mark Cushman, Ph.D., at Purdue University, was the result of an "unexpected undesired reaction," as he put it, that occurred as he attempted to synthesize the anti-leukemia agent nitidine chloride. Instead of discarding it, Cushman placed the indenoisoquinoline compound in the NCI-60 database, where it sat untouched for 18 years, until he received a phone call from Paull.
Cushman immediately set to work making indenoisoquinoline analogs—400–500 of them—which he sent to NCI for Pommier's group to test against purified topo I and in cell culture for structureactivity relationships. The data led Pommier and Cushman to focus on the two most promising candidates, which are now on the verge of entering the clinic for the first time. "At this point, we've done preclinical and toxicology work, and the clinical protocols have been written," said Pommier.
But advancing these compounds from the chemistry lab to the clinic would not have been possible without the DTP, which as a mission takes lead compounds that show promise in cell culture and puts them through the many hurdles of animal experiments and formulations that must be cleared before first-in-human trials. For example, the academic synthesis protocols that Cushman develops may bear scant relation to the synthesis processes necessary for the high-volume commercial manufacturing steps that a pharmaceutical company must employ. Similarly, the drugs that Pommier tests in vitro are all dissolved in DMSO, which is toxic to human beings, and so must be assessed for solubility in non-toxic solvents as well. The DTP has even enabled the development of a biomarker for topo I inhibition that research clinicians will use in their clinical trials, the phosphorylation of histone γ-H2AX. This biomarker was first associated with DNA damage by another CCR investigator, William Bonner, Ph.D. Another NCI colleague, James Doroshow, M.D., Director of NCI's Division of Cancer Treatment and Diagnosis, collaborates with CCR's Laboratory of Molecular Pharmacology and has been a key player in the development team leading to the clinical evaluation of the indenoisoquinolines at the NIH clinical center.
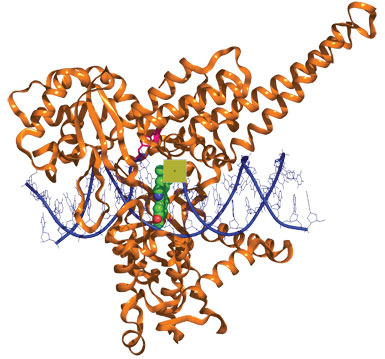
Indenoisoquinolines (green) glue complexes of topoisomerase I (brown) and DNA (blue) together.
Pommier and Postdoctoral Fellow Thomas Dexheimer, Ph.D., continue to collaborate with Cushman to test new potential topo I inhibitors. "When you have one target, you want to have more than one type of drug," said Pommier. "Even drugs in the same family, such as irinotecan and topotecan, have different clinical profiles. We're making the assumption, but I think it is likely to be the case, that the indenoisoquinolines are going to have a different clinical profile from any of the camptothecins. And we have many arguments to say why they have advantages, but the proof will become apparent when we give these compounds to patients."
Nature Plus Nurture: The Consortium Approach
Citing the examples of paclitaxel and camptothecin, Pommier is convinced that Nature has many more hidden treasures that could benefit mankind's health: "Nature has taken a long time to optimize for us," he said. "Although we now have powerful methods for visualizing and predicting compounds' structural features and binding activities, rational drug design is not the only way forward." Rather, screening and rational drug design are complementary parts of an overall drug discovery strategy that Pommier and his colleagues are using to go after another cancer target, the DNA repair enzyme Tyr-DNA-PDE, or TDP. TDP repairs the stalled DNA replication caused by topo I inhibitors, so cells that are missing TDP are hypersensitive to topo I inhibitors.
Pommier is convinced that Nature has many more hidden treasures that could benefit mankind's health.
Because TDP had no known inhibitors, Christophe Marchand, Ph.D., a Staff Scientist in Pommier's group, spearheaded high-throughput screening against TDP in collaboration with the NIH Chemical Genomics Center (NCGC) up the road in Gaithersburg, Md. Although Marchand had already developed an assay for the Pommier laboratory's in-house screening system when they began their collaboration, he needed to reoptimize it for the NCGC, more or less on his own. "We got lucky," he said of the success of his early optimization attempts. After less than a year, NCGC was convinced that it could screen its entire compound library of over 300,000 compounds against Marchand's TDP inhibitor assay, a screen that was completed in the first week of June 2008.
The TDP project now includes more than just Pommier's group and the NCGC. NCI's Chemical Biology Consortium has since taken an interest in the work and set up an entire team of investigators, supported by dedicated project managers, to promote the development of TDP inhibitors "from bench to bedside." Across NCI, more than 20 investigators meet regularly to share data and plan new experiments, including using synthetic chemistry to design better inhibitors based on the structural analysis of lead compounds (see "SCSORS Takes the Lead").
Marchand counts the success of this project to date among his proudest achievements and is excited about the collaboration and the opportunities afforded by a large consortium in overcoming practical obstacles. "For the first time, I have the feeling that we are surfing on big waves."
"The resources are amazing, although they aren't always connected up as well as we'd like," Pommier noted when describing the path he took to establish a collaboration with the NCGC. "The NCI is a powerful place for this kind of work."
 Yves Pommier, M.D., Ph.D.
Yves Pommier, M.D., Ph.D. Christophe Marchand, Ph.D.
Christophe Marchand, Ph.D. Thomas Dexheimer, Ph.D.
Thomas Dexheimer, Ph.D.

