News
Resistance Is Futile:
Examining the Causes of Cisplatin Resistance in Cancer Cells
The platinum-based anti-cancer drug cisplatin is a basic component of today’s portfolio of chemotherapy drugs, being widely used to treat bladder, ovarian, testicular, lung, and other common cancers. Unfortunately, cisplatin-treated tumors frequently become resistant, for reasons that remain unclear. CCR researchers Michael M. Gottesman, M.D., Chief of the Laboratory of Cell Biology; Xing-Jie Liang, Ph.D., former CCR Fellow and current Director, Laboratory of Nanomedicine and Nanosafety, National Center for Nanoscience and Technology, Beijing, China; and colleagues set out to examine the root causes of cisplatin resistance. Their results, published in the September 15, 2008, issue of Molecular Cancer Research, isolate one of the many factors contributing to cellular resistance to cisplatin.
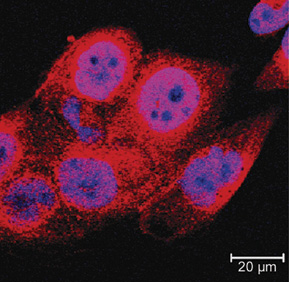
Increased levels of SIRT1 (red), a histone deacetylase linked to cell metabolism, contribute to cisplatin resistance (CP-r) in tumor cells. By understanding SIRT1 and structural and functional changes to the mitochondria in CP-r cells, researchers hope to develop strategies to prevent cisplatin resistance and maintain the drug’s efficacy.
Previous research by the team showed that cisplatin-resistant (CP-r) cells grow more slowly and consume fewer nutrients than those that are cisplatin-sensitive (CP-s). Because tumors can change their metabolism as a survival strategy when nutrients are scarce, the researchers decided to focus on examining the metabolic changes that occur in CP-r tumor cells.
The researchers generated CP-r cells by exposing liver and epidermoid carcinoma cell lines to cisplatin in vitro. Comparing these to CP-s cancer cell lines, the researchers found significant reductions in the CP-r cells’ use of glucose (4–5 fold) and oxygen (30–60 percent). Mitochondria, the cell’s metabolic engines, rely on oxygen and glucose to produce energy; therefore, the team also compared the mitochondria in CP-r and CP-s cells. They found that the mitochondria of CP-r cells are smaller and both functionally and structurally altered compared to CP-s cells.
Based on these basic differences, the team looked next at SIRT1, a histone deacetylase known to play a pivotal role in regulating cellular metabolism and responses to nutrient restriction. Measuring the SIRT1 levels in both cisplatin-resistant and -sensitive cells, the researchers discovered that the CP-r cells overexpressed SIRT1. Introduction of SIRT1 into cells increased cisplatin resistance, while knocking down SIRT1 expression using RNAi decreased resistance.
This direct link between the cisplatin exposure, resistance, and SIRT1 levels suggests that SIRT1 plays a key role in cisplatin resistance, a valuable insight that can help researchers develop strategies that decrease the potential for resistance and increase the efficacy of this important anti-cancer drug.
Building a Real-Time Resistance Fighter
More than a dozen multidrug-resistant cell lines, like the CP-r cell line studied by Gottesman and Liang, have been developed by the Gottesman laboratory and licensed to over 40 pharmaceutical or biotech companies over the past 20 years. The laboratory’s research on changes in cancer cells’ handling of drugs as a way to survive therapy, as well as the work of many other labs, has implicated 380 resistance-related genes, which the team is now assembling onto a “gene chip.” Their goal is to develop a clinically relevant diagnostic tool that can be used to rapidly detect drug resistance in real time as it arises.










