
Andrew J. Griffith, M.D., Ph.D.
Chief
Otolaryngology Branch
Chief
Molecular Biology and Genetics Section
Otolaryngology Branch
NIDCD/NIH
5 Research Court, 1A-13
Rockville, MD 20850
Phone: (301) 496-1960
Fax: (301) 402-7580
E-mail: griffita@nidcd.nih.gov
Dr. Griffith received M.D. and Ph.D. degrees from Yale University. He completed an Otolaryngology-Head and Neck Surgery residency at the University of Michigan, where he also received fellowship training in the laboratory of Dr. Miriam Meisler in the Department of Human Genetics.
On this page:
Otolaryngology Branch

Dr. Griffith examines a CT scan Basic molecular biology and genetic research is carried out in the Molecular Biology and Genetics Section. Audiology Unit research activities include audiological and vestibular assessment of human subjects participating in clinical research protocols of the Otolaryngology Branch, the NIDCD, or other NIH Institutes and Centers.
Dr. Hung Jeff Kim is responsible for Neuro-Otology evaluations, treatment, and research. Ms. Julie Muskett is the Genetic Counselor for the NIDCD. Her responsibilities include coordinating clinical research protocols in the Otolaryngology Branch and the Laboratory of Molecular Genetics, and genetic counseling of subjects participating in NIDCD research studies.
Subjects Needed for Research Studies
The Otolaryngology Branch is seeking research subjects from families to participate in research studies in the following areas:
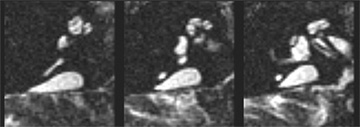
Axial T2-weighted fast spin echo MRI images of the right temporal bone showing enlargement of the endolymphatic system (known as EVA) when visualized on CT scans. Personnel
Hung Jeff Kim, M.D., Staff Neuro-Otologist (Send e-mail | View photo)
Julie Muskett, M.S., Genetic Counselor (Send e-mail | View photo)
Zelia Pulliam, Office Manager (Send e-mail | View photo)
Top
Molecular Biology and Genetics Section
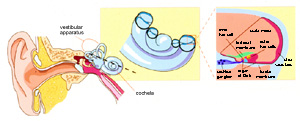
Schematic illustration of the mammalian auditory system (from Griffith and Friedman, Nature Genetics, 1999). View larger image. Research Statement
Our laboratory identifies and characterizes genes, molecules, and mechanisms underlying hearing and hereditary hearing loss. We use molecular biologic and genetic approaches, human and mouse models, as well as heterologous cell culture expression systems. A variety of techniques—including in situ hybridization, immunohistochemistry, RT-PCR, Western and Northern blotting, and immunoprecipitation—are used to analyze gene and protein expression, function, and interactions.
Current Areas of InterestWe identified a novel gene, TMC1, which underlies dominant DFNA36 and recessive DFNB7/B11 deafness in humans. Dominant and recessive mutations in the mouse Tmc1 gene underlie deafness in the Beethoven (Bth) and deafness (dn) mouse mutants, respectively, which exhibit rapid degeneration of the neurosensory hair cells of the cochleae. TMC1 protein has no sequence similarities to proteins or domains of known function, but has six transmembrane domains whose topologic organization suggests a role as an ion channel or transporter. There are seven other members of the mammalian TMC gene family, and we are using mutant mouse models to identify the function(s) of Tmc1 and these other Tmc genes.
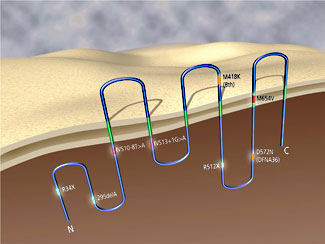
Schematic illustration of TMC1 showing predicted transmembrane topology and human and mouse mutations causing deafness. View larger image. A second project is the molecular genetic analysis of human hearing loss associated with enlargement of the vestibular aqueduct (EVA). EVA is the most commonly observed inner ear malformation in children with hearing loss. In many cases EVA is associated with mutations in the Pendred syndrome gene PDS/SLC26A4, which encodes an integral membrane protein that is thought to transport or exchange chloride, iodide, bicarbonate, or other bases in the inner ear.
Lab Personnel
Yoshiyuki Kawashima, M.D., Visiting Scientist (Send e-mail | View photo)
Kiyoto Kurima, Ph.D., Staff Scientist (Send e-mail | View photo)
Valentina Labay, Ph.D., Visiting Fellow (Send e-mail | View photo)
Kelly Monahan, B.S., Postbaccalaureate IRTA Fellow (Send e-mail | View photo)
Lab Photos
Click for larger image
Top
Audiology Unit
Research Statement
The primary research interest of the Audiology Unit is the pathogenesis and manifestations of hereditary hearing and balance disorders, and the correlation of distinctive auditory and vestibular phenotypes with underlying molecular genotypes. Additional activities include studies of hearing loss associated with noise or other ototoxins, and inflammatory, neoplastic or infectious processes. We use a comprehensive battery of auditory and vestibular measures that includes otoacoustic emissions, wideband acoustic reflectance, auditory processing evaluation, psychoacoustic measures of pitch perception, auditory and vestibular evoked potentials, videonystagmography, rotary vestibular chair testing, and computerized dynamic platform posturography.
Current Areas of Interest
The Audiology Unit is studying the hearing and balance manifestations of several different forms of syndromic and nonsyndromic human hereditary hearing loss, and correlating clinical features with underlying gene mutations. Many of our studies are part of NICHD, NCI, NIAMS, NINDS, and NHGRI clinical research protocols on a variety of chromosomal and syndromic disorders affecting hearing and balance.
Additional projects include: (1) hearing safety during clinical and research procedures such as magnetic resonance imaging and transcranial magnetic stimulation that involve exposure to high noise levels; (2) effectiveness of various types of hearing protection and the cumulative impact of repeated exposure associated with these procedures; (3) heritability of auditory processing skills, in conjunction with the Laboratory of Molecular Genetics, NIDCD.
Personnel
Carmen Brewer, Ph.D., Chief of Audiology (Send e-mail | View photo)
Kelly King, Au.D., Research Assistant (Send e-mail | View photo)
Christopher Zalewski, M.S., Staff Audiologist (Send e-mail | View photo)
Top
Selected Publications
2009
- Jones JL, Zalewski C, Brewer C, Lucker J, Drayna D. Widespread auditory deficits in tune deafness. Ear Hear. 2009 Feb;30(1):63-72.
- Khan SG, Oh KS, Emmert S, Imoto K, Tamura D, Digiovanna JJ, Shahlavi T, Armstrong N, Baker CC, Neuburg M, Zalewski C, Brewer C, Wiggs E, Schiffmann R, Kraemer KH. XPC initiation codon mutation in xeroderma pigmentosum patients with and without neurological symptoms. DNA Repair (Amst). 2009 Jan 1;8(1):114-25. Epub 2008 Nov 14.
2008
- Ahmed ZM, Masmoudi S, Kalay E, Belyantseva IA, Mosrati MA, Collin RW, Riazuddin S, Hmani-Aifa M, Venselaar H, Kawar MN, Tlili A, van der Zwaag B, Khan SY, Ayadi L, Riazuddin SA, Morell RJ, Griffith AJ, Charfedine I, Caylan R, Oostrik J, Karaguzel A, Ghorbel A, Riazuddin S, Friedman TB, Ayadi H, Kremer H. Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans.Nat Genet 2008 Nov;40(11):1335-40. Epub 2008 Oct 26.
- Braun A, McArdle J, Jones J, Nechaev V, Zalewski C, Brewer C, Drayna D. Tune deafness: processing melodic errors outside of conscious awareness as reflected by components of the auditory ERP. Public Library of Science (PLoS) One 11;3(6):e2349,2008
- Driver EC, Pryor SP, Hill P, Turner J, Rüther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. Journal of Neuroscience 16;28(29):7350–8, 2008.
- Peters LM, Fridell RA, Boger ET, San Agustin TB, Madeo AC, Griffith AJ, Friedman TB, Morell RJ. A locus for autosomal dominant progressive non-syndromic hearing loss, DFNA27, is on chromosome 4q12-13.1. Clinical Genetics 73(4):367–72, 2008.
- Lonser RR, Baggenstos M, Kim HJ, Butman JA, Vortmeyer AO. The vestibular aqueduct: site of origin of endolymphatic sac tumors. Journal of Neurosurgery 108(4):751–6, 2008.
- Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO 3rd, Gahl WA, Introne WJ. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008 Feb 7;358(6):592–604.
- Kalueff AV, Ishikawa K, Griffith AJ. Anxiety and otovestibular disorders: Linking behavioral phenotypes in men and mice. Behavioural Brain Research 186(1):1–11, 2008.
- Labay V, Garrido G, Madeo AC, Nance WE, Friedman TB, Friedman PL, Del Castillo I, Griffith AJ. Haplogroup analysis supports a pathogenic role for the 7510T>C mutation of mitochondrial tRNA(Ser(UCN)) in sensorineural hearing loss. Clinical Genetics 73(1):50–4, 2008.
2007
- Doherty ES, Lacbawan F, Hadley DW, Brewer C, Zalewski C, Kim HJ, Solomon B, Rosenbaum K, Domingo DL, Hart TC, Brooks BP, Immken L, Lowry RB, Kimonis V, Shanske AL, Jehee FS, Bueno MR, Knightly C, McDonald-McGinn D, Zackai EH, Muenke M. Muenke syndrome (FGFR3-related craniosynostosis): expansion of the phenotype and review of the literature. American Journal of Medical Genetics. Part A 2007 Dec 15;143(24):3204–15, 2007.
- King KA, Makishima T, Zalewski CK, Bakalov VK, Griffith AJ, Bondy CA, Brewer CC. Analysis of Auditory Phenotype and Karyotype in 200 Females with Turner Syndrome. Ear Hear 28(6):831–841, 2007.
- Kitajiri SI, McNamara R, Makishima T, Husnain T, Zafar A, Kittles R, Ahmed Z, Friedman T, Riazuddin S, Griffith A. Identities, frequencies and origins of TMC1 mutations causing DFNB7/B11 deafness in Pakistan. Clinical Genetics 72(6):546–50, 2007.
- Jagannathan J, Lonser RR, Stanger RA, Butman JA, Vortmeyer AO, Zalewski CK, Brewer C, Surowicz C, Kim HJ. Cochlear implantation for hearing loss associated with bilateral endolymphatic sac tumors in von Hippel-Lindau disease. Otology & Neurotology 28(7):927–30, 2007.
- Butman JA, Kim HJ, Baggenstos M, Ammerman JM, Dambrosia J, Patsalides A, Patronas NJ, Oldfield EH, Lonser RR. Mechanisms of Morbid Hearing Loss Associated With Tumors of the Endolymphatic Sac in von Hippel-Lindau Disease. JAMA: The Journal of the American Medical Association 298(1):41–8, 2007.
- Makishima T, Madeo AC, Brewer CC, Zalewski CK, Butman JA, Sachdev V, Arai AE, Holbrook BM, Rosing DR, Griffith AJ. Nonsyndromic Hearing Loss DFNA10 and a Novel Mutation of EYA4: Evidence for Correlation of Normal Cardiac Phenotype with Truncating Mutations of the Eya Domain. American Journal of Medical Genetics 143(14):1592–8, 2007.
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of Fibroblast Growth Factor Receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Developmental Dynamics 236(7):1905–17, 2007.
- Jagannathan J, Butman JA, Lonser RR, Vortmeyer AO, Zalewski CK, Brewer C, Oldfield EH, Kim HJ. Endolymphatic sac tumor demonstrated by intralabyrinthine hemorrhage. Case report. Journal of Neurosurgery 107(2):421–5, 2007.
- Ain Q, Nazli S, Riazuddin S, Jaleel AU, Riazuddin SA, Zafar AU, Khan SN, Husnain T, Griffith AJ, Ahmed ZM, Friedman TB, Riazuddin S. The autosomal recessive nonsyndromic deafness locus DFNB72 is located on chromosome 19p13.3. Human Genetics 122(5):445–50, 2007.
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL. Deafness and Stria Vascularis Defects in S1P2 Receptor-null Mice. The Journal of Biological Chemistry 282(14):10690–6, 2007.
- Morell RJ, Brewer CC, Ge D, Snieder H, Zalewski CK, King KA, Drayna D, Friedman TB. A twin study of auditory processing indicates that dichotic listening ability is a strongly heritable trait. Human Genetics 122(1):103–11, 2007.
- Kitajiri S, Makishima T, Friedman TB, and Griffith AJ. A novel mutation at the DFNA36 hearing loss locus reveals a critical function and potential genotype-phenotype correlation for amino acid-572 of TMC1. Clinical Genetics 71:148–152, 2007.
- Ries M, Kim HJ, Zalewski CK, Mastroianni MA, Moore DF, Brady RO, Dambrosia JM, Schiffmann R, and Brewer CC. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain 130:143–50, 2007.
2006
- Stepanyan R, Belyantseva IA, Griffith AJ, Friedman TB, and Frolenkov GI. Auditory mechanotransduction in the absence of functional myosin-XVa. The Journal of Physiology. 576:801–808, 2006.
- Ondrey FG, Moldestad E, Mastroianni MA, Pikus A, Sklare D, Vernon E, Nusenblatt R, Smith J. Sensorineural hearing loss in Vogt-Koyanagi-Harada syndrome. Laryngoscope 116(10):1873–6, 2006.
- Griffith AJ, Yang Y, Pryor SP, Park HJ, Jabs EW, Nadol JB Jr, Russell LJ, Wasserman DI, Richard G, Adams JC, and Merchant SN. Cochleosaccular dysplasia associated with Connexin 26 deafness in KID syndrome. Laryngoscope 116:1404–1408, 2006.
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, and Friedman TB. The tip-link antigen TB, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. Journal of Neuroscience. 26:7022–7034, 2006.
- Noguchi Y, Kurima K, Makishima T, de Angelis MH, Fuchs H, Frolenkov G, Kitamura K, and Griffith AJ. Multiple quantitative trait loci modify cochlear hair cell degeneration in the Beethoven (Tmc1Bth) mouse model of progressive hearing loss DFNA36. Genetics 173:2111–2119, 2006.
- Madeo AC, Pryor SP, Brewer C, Zalewski C, King K, Butman JA, Yang Y, Park HJ, and Griffith AJ, 2006. Pendred syndrome. Seminars in Hearing. 27:160–170.
- Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O'Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesle RA, Hoffmann SC, Holland SM, Butman JA, and Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. New England Journal of Medicine. 355:581–92, 2006.
2005
- Rose PS, Levy HP, Liberfarb RM, Davis J, Szymko-Bennett Y, Rubin BI, Tsilou E, Griffith AJ, Francomano CA. Stickler syndrome: clinical characteristics and diagnostic criteria. American Journal of Medical Genetics Part A 138(3):199–207, 2005.
- Vogel TW, Vortmeyer AO, Lubensky IA, Lee YS, Furuta M, Ikejiri B, Kim HJ, Lonser RR, Oldfield, Zhuang Z. Coexpression of erythropoietin and its receptor in endolymphatic sac tumors. Journal of Neurosurgery 103(2):284–8, 2005.
- Pryor SP, Demmler GJ, Madeo AC, Yang Y, Zalewski CK, Brewer CC, Butman JA, Fowler KB, Griffith AJ. Investigation of the role of congenital cytomegalovirus infection in the etiology of enlarged vestibular aqueducts. Archives of Otolaryngology—Head & Neck Surgery 131(5):388–92, 2005.
- Schultz JM, Yang Y, Caride AJ, Filoteo AG, Penheiter AR, Lagziel A, Morell RJ, Mohiddin SA, Fananapazir L, Madeo AC, Penniston JT, Griffith AJ. Modification of human hearing loss by plasma-membrane calcium pump PMCA2. New England Journal of Medicine 352(15):1557–64, 2005. Erratum in New England Journal of Medicine 352(22):2362, 2005.
- Kim HJ, Butman JA, Brewer C, Zalewski C, Vortmeyer AO, Glenn G, Oldfield EH, Lonser RR. Tumors of the endolymphatic sac in patients with von Hippel-Lindau disease: implications for their natural history, diagnosis, and treatment. Journal of Neurosurgery 102(3):503–12, 2005.
- Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, Nance WE, Yang Y, Zalewski CK, Brewer CC, Butman JA, Griffith AJ. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. Journal of Medical Genetics 42(2):159–65, 2005.
- Park HJ, Lee SJ, Jin HS, Lee JO, Go SH, Jang HS, Moon SK, Lee SC, Chun YM, Lee HK, Choi JY, Jung SC, Griffith AJ, Koo SK. Genetic basis of hearing loss associated with enlarged vestibular aqueducts in Koreans. Clinical Genetics 67(2):160–5, 2005.
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nature Cell Biology 7(2):148–56, 2005. Epub 2005 Jan 16.
2004
- Makishima T, Kurima K, Brewer CC, Griffith AJ. Early onset and rapid progression of dominant nonsyndromic DFNA36 hearing loss. Otology & Neurotology 25(5):714–9, 2004.
- Ahmed ZM, Li XC, Powell SD, Riazuddin S., Young TL, Ramzan K., Ahmad Z., Luscombe S., Dhillon K., MacLaren L., Ploplis B., Shotland LI, Ives E., Riazuddin S., Friedman TB, Morell RJ, Wilcox ER. Characterization of a new full length TMPRSS3 isoform and identification of mutant alleles responsible for nonsyndromic recessive deafness in Newfoundland and Pakistan. BMC Medical Genetics 5:24, 2004.
- Naz S, Griffith AJ, Riazuddin S, Hampton LL, Battey JF Jr, Khan SN, Riazuddin S, Wilcox ER, Friedman TB. Mutations of ESPN cause autosomal recessive deafness and vestibular dysfunction. Journal of Medical Genetics 41(8):591–5, 2004.
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic Insights Into the Morphogenesis of Inner Ear Hair Cells (available as PDF; get Adobe Reader) Nature Reviews Genetics 5:489–98, 2004. Reproduced with permission from Nature Reviews Genetics; Macmillan Magazines Ltd.
- Lao CD, Backoff P, Shotland LI, McCarty D, Eaton T, Ondrey FG, Viner JL, Spechler SJ, Hawk ET, Brenner DE. Irreversible ototoxicity associated with difluoromethylornithine. Cancer Epidemiology, Biomarkers & Prevention 13:1250–2, 2004.
- Lonser RR, Kim HJ, Butman JA, Vortmeyer AO, Choo DI, Oldfield EH. Tumors of the endolymphatic sac in von Hippel-Lindau disease. New England Journal of Medicine 350:2481–6 EH, 2004.
- Shpargel KB, Makishima T, Griffith AJ. Col11a1 and Col11a2 mRNA expression in the developing mouse cochlea: implications for the correlation of hearing loss phenotype with mutant type XI collagen genotype. Acta Oto-Laryngologica 124(3):242–8, 2004.
- Mohiddin SA, Ahmed ZM, Griffith AJ, Tripodi D, Friedman TB, Fananapazir L, Morell RJ. Novel association of hypertrophic cardiomyopathy, sensorineural deafness, and a mutation in unconventional myosin VI (MYO6). Journal of Medical Genetics 41(4):309–14, 2004.
- Choo D, Shotland L, Mastroianni M, Glenn G, van Waes C, Linehan WM, Oldfield EH. Endolymphatic sac tumors in von Hippel-Lindau disease. Journal of Neurosurgery 100(3):480–7, 2004.
2003
- Shotland LI, Mastroianni MA, Choo DL, Szymko-Bennett YM, Dally LG, Pikus AT, Sledjeski K, Marques A. Audiologic manifestations of patients with post-treatment Lyme disease syndrome. Ear and Hearing. 24:508–517, 2003.
- Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annual Review of Genomics and Human Genetics 4:341–402, 2003.
- Ahmed ZM, Riazuddin S, Ahmad J, Bernstein SL, Guo Y, Sabar MF, Sieving P, Riazuddin S, Griffith AJ, Friedman TB, Belyantseva IA, Wilcox ER. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Human Molecular Genetics 12(24):3215–23, 2003. Epub 2003 Oct 21.
- Belyantseva IA, Labay V, Boger ET, Griffith AJ, Friedman TB. Stereocilia: the long and the short of it. Trends in Molecular Medicine 9(11):458–61, 2003.
- Ness SL, Ben-Yosef T, Bar-Lev A, Madeo AC, Brewer CC, Avraham KB, Kornreich R, Desnick RJ, Willner JP, Friedman TB, Griffith AJ. Genetic homogeneity and phenotypic variability among Ashkenazi Jews with Usher syndrome type III. Journal of Medical Genetics 40(10):767–772, 2003.
- Yan D, Park HJ, Ouyang XM, Pandya A, Doi K, Erdenetungalag R, Du LL, Matsushiro N, Nance WE, Griffith AJ, Liu XZ. Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in east Asians. Human Genetics 114(1):44–50, 2003. Epub 2003 Sep 18.
- Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis small star, filled. Genomics 82(3):300–308, 2003.
- Friedman TB, Schultz JM, Ben-Yosef T, Pryor SP, Lagziel A, Fisher RA, Wilcox ER, Riazuddin S, Ahmed ZM, Belyantseva And IA, Griffith AJ. Recent advances in the understanding of syndromic forms of hearing loss. Ear and Hearing 24(4):289–302, 2003.
- Naz S, Alasti F, Mowjoodi A, Riazuddin S, Sanati MH, Friedman TB, Griffith AJ, Wilcox ER, Riazuddin S. Distinctive audiometric profile associated with DFNB21 alleles of TECTA. Journal of Medical Genetics 40(5):360–363, 2003.
- Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, Mohiddin SA, Fananapazir L, Caruso RC, Husnain T, Khan SN, Riazuddin S, Griffith AJ, Friedman TB, Wilcox ER. Mutations of MYO6 are associated with recessive deafness, DFNB37. American Journal of Human Genetics 72(5):1315–1322, 2003.
- Ben-Yosef T, Ness SL, Madeo AC, Bar-Lev A, Wolfman JH, Ahmed ZM, Willner JP, Desnick RJ, Avraham KB, Ostrer H, Oddoux C, Griffith AJ, and Friedman TB. A mutation of PCDH15 among Ashkenazi Jews with the type 1 Usher syndrome. New England Journal of Medicine 348:1664–1670, 2003.
- Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, Kim HN, Moon SK, Abe S, Tukamoto K, Riazuddin S, Kabra M, Erdenetungalag R, Radnaabazar J, Khan S, Pandya A, Usami SI, Nance WE, Wilcox ER, Riazuddin S, Griffith AJ. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. Journal of Medical Genetics 40(4):242–248, 2003.
- Liberfarb RM, Levy HP, Rose PS, Wilkin DJ, Davis J, Balog JZ, Griffith AJ, Szymko-Bennett YM, Johnston JJ, Francomano CA, Tsilou E, Rubin BI. The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genetics in Medicine 5(1):21–7, 2003. Review. Erratum in: Genetics in Medicine 5(6):478, 2003.
- Szymko-Bennett YM, Kurima K, Olsen B, Seegmiller R, Griffith AJ. Auditory function associated with Col11a1 haploinsufficiency in chondrodysplasia (cho) mice. Hearing Research 175(1–2):178–82, 2003.
2002
- Peters LM, Anderson DW, Griffith AJ, Grundfast KM, San Agustin TB, Madeo AC, Friedman TB, Morell RJ. Mutation of a transcription factor, TFCP2L3, causes progressive autosomal dominant hearing loss, DFNA28. Human Molecular Genetics 11(23):2877–2885, 2002.
- Griffith AJ, Szymko YM, Kaneshige M, Quinonez R, Kaneshige K, Mastroianni MA, Kelley MW, Cheng S. Knock-in mouse model for Resistance to Thyroid Hormone (RTH): an RTH mutation in the thyroid hormone receptor beta gene disrupts cochlear morphogenesis. Journal of the Association for Research in Otolaryngology 3(3):279–288, 2002.
- Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, Griffith AJ, Friedman TB, Smith RJ, Wilcox ER. Mutations in a novel gene, TMIE, are associated with hearing loss at the DFNB6 locus. American Journal of Human Genetics 71(3):632–636, 2002.
- Ahmed, ZM, Smith TN, Riazuddin S, Makishima T, Ghosh M, Bokhari S, Menon PS, Deshmukh D, Griffith AJ, Riazuddin S, Friedman TB, Wilcox ER. Nonsyndromic recessive deafness DFNB18 and Usher syndrome type 1C are allelic mutations of USH1C. Human Genetics 110(6):527–531, 2002.
- Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, de Angelis MH, Avraham KB, Steel KP. Beethoven, a mouse model for dominant progressive hearing loss DFNA36. Nature Genetics 30:257–258, 2002.
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PSN, Deshmukh D, Oddoux C, Ostrer H, Khan S, Riazuddin S, Hampton LL, Deininger PL, Battey JF Jr, Keats BJB, Wilcox ER, Friedman TB, Griffith AJ. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nature Genetics 30:277–284, 2002.
- Szymko-Bennett YM, Russell LJ, Bale SJ, Griffith AJ. Auditory manifestations of Keratitis-Ichthyosis-Deafness (KID) syndrome. Laryngoscope 112(2):272–80, 2002.
Top |
 |
|