News
Small Molecule, Big Impact:
The Efficacy of Lapatinib in Breast Cancer Metastases in the Brain
The treatment of brain metastases linked to breast tumors overexpressing the protein HER2 represents a growing unmet medical need. HER2- positive tumors account for approximately 20 to 25 percent of all breast cancers, and 35 percent of HER2 metastatic patients will experience outgrowth to the brain. Treatment options for brain metastases are currently limited to radiotherapy, neurosurgery, and steroids, largely due to a lack of drug therapies capable of crossing the blood-brain barrier. For example, trastuzumab (Herceptin®), a monoclonal antibody against HER2, is too large to reach tumors that have spread to the brain.
A team of researchers led by Visiting Postdoctoral Fellow Brunilde Gril, Ph.D.; Staff Scientist Diane Palmieri, Ph.D.; and Principal Investigator Patricia Steeg, Ph.D., of the Women’s Cancers Section in CCR’s Laboratory of Molecular Pharmacology, recently completed a study demonstrating that the kinase inhibitor lapatinib (Tykerb®) effectively inhibited the growth of HER2-positive breast cancer cells that metastasize to the brain. The study results appear in the August 6, 2008, issue of the Journal of the National Cancer Institute.
Lapatinib is a small-molecule drug that blocks both HER2 and another protein, epidermal growth factor receptor (EGFR). Breast cancers that overexpress HER2 and/ or EGFR are more likely to metastasize to the brain. Because only small, lipophilic drugs can cross the blood-brain barrier, lapatinib may be an ideal candidate for the treatment of such cancers.
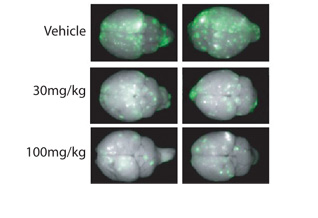
A lack of drug therapies capable of crossing the blood-brain barrier leaves HER2-positive breast cancer patients who develop brain metastases with limited treatment options. As a small-molecule, lipophilic drug, lapatinib successfully crosses the blood-brain barrier to reduce HER2-positive brain metastases (green) in preclinical models.
To test this hypothesis, Steeg, Palmieri, Gril, and their colleagues treated brain-seeking breast cancer cells with lapatinib in vitro. The results demonstrated that the drug inhibited the activation of both EGFR and HER2 pathways, thus restricting cell proliferation and spread. Furthermore, cell lines expressing high levels of both EGFR and HER2 were 30 percent more vulnerable to the drug than lines that expressed high levels of only one of the receptors.
The researchers then turned to in vivo models to confirm their results. Mice injected with HER2-positive breast cancer cells developed twice as many large metastases as those injected with cells expressing normal levels of the protein. While lapatinib was again significantly more effective against tumor cells that overexpressed both HER2 and EGFR, it could not completely prevent metastatic development.
Nevertheless, utilizing lapatinib as an adjuvant or preventive therapy for HER2-overexpressing breast cancer warrants further clinical investigation. Neurosurgery and radiotherapy may continue to offer the best treatment options for large metastases in the brain, but the study results demonstrate that lapatinib could be the first small-molecule therapy to successfully prevent the progression of micrometastases in a preclinical model.