 |

Drug Combination Cuts Risk of Advanced Colon Polyps
Results from a phase III clinical trial indicate that low doses of two chemopreventive agents, an anti-inflammatory and an experimental compound, are highly effective at preventing the recurrence of the lesions that are often precursors to colorectal cancer.
Trial results presented yesterday at the American Association for Cancer Research (AACR) annual meeting in San Diego, CA, showed that, compared with participants in the placebo arm, those treated with a combination of the investigational compound difluoromethylornithine (DFMO) and the anti-inflammatory agent sulindac had their risk of colon polyp recurrence reduced by 70 percent. More important, the results showed that the treatment was most effective in preventing the recurrence of the highest-risk polyps, advanced adenomas, demonstrating a 92 percent reduction.
Read more 


Mutant Gene Linked to Aggressive Leukemia
Researchers have identified a common genetic change in Philadelphia chromosome (BCR-ABL1)-positive acute lymphoblastic leukemia (ALL), an aggressive leukemia that carries a poor prognosis. The change alters the gene IKZF1, which produces the protein Ikaros, and appears to be an important lesion in this ALL subtype, the researchers reported online in the April 13 Nature.
Ikaros plays a key role in regulating the normal development of lymphocytes. The IKZF1 gene alterations were detected as part of an ongoing effort to identify genetic abnormalities in ALL. Dr. James Downing of St. Jude Children's Research Hospital and his colleagues analyzed DNA copy number changes in 304 ALL cases, including 21 children and 22 adults with BCR-ABL1-positive disease.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Drug Combination Cuts Risk of Advanced Colon Polyps
Results from a phase III clinical trial indicate that low doses of two chemopreventive agents, an anti-inflammatory and an experimental compound, are highly effective at preventing the recurrence of the lesions that are often precursors to colorectal cancer.
Trial results presented yesterday at the American Association for Cancer Research (AACR) annual meeting in San Diego, CA, showed that, compared with participants in the placebo arm, those treated with a combination of the investigational compound difluoromethylornithine (DFMO) and the anti-inflammatory agent sulindac had their risk of colon polyp recurrence reduced by 70 percent. More important, the results showed that the treatment was most effective in preventing the recurrence of the highest-risk polyps, advanced adenomas, demonstrating a 92 percent reduction.
"This is the most positive trial in chemoprevention in the last 20 years," says the trial's lead investigator Dr. Frank L. Meyskens from the Chao Family Comprehensive Cancer Center at the University of California, Irvine. "It should raise great hope that we'll be able to use chemoprevention in appropriate at-risk groups."
The trial was stopped early, following a recommendation by its Data Safety Monitoring Board, because the trial's primary goals had been met. Although 375 patients were randomly assigned to the placebo or intervention arms, the final analysis was based on data from 267 patients for whom the most complete information was available. All patients in the trial had previously had colorectal adenomas (3 mm or larger) removed, and the study treatment lasted for 36 months.
"This is a phase III study that demonstrates a lot of potential for this drug combination to decrease cancer risk," says Dr. Eva Szabo from NCI's Division of Cancer Prevention.
Data on cancer incidence are not yet available from the trial. "However," Dr. Szabo continues, "we understand the biology of polyp progression to colon cancer quite well." Given the decrease in adenoma recurrence seen in the study, there is a very strong likelihood of a true cancer prevention effect, she notes.
Originally developed by a French pharmaceutical company, DFMO inhibits a cell's synthesis of molecules called polyamines by blocking the function of an enzyme known as ornithine decarboxylase.
Based on data from laboratory and animal model studies, explains Dr. Meyskens - who, along with Dr. Eugene Gerner from the Arizona Cancer Center, has been studying DFMO since the early 1980s - the combination of DFMO and sulindac makes sense for cancer prevention.
"They affect two different pathways, both of which drive proliferation," he says.
Sulindac was an early member of the class of drugs known as nonsteroidal anti-inflammatory drugs, or NSAIDs. That same class also includes celecoxib (Celebrex), a COX-2 inhibitor that has had mixed results in other large adenoma recurrence prevention trials. It decreased adenoma recurrence but also increased the risk of serious cardiac events.
However, Dr. Meyskens cautions, "I don't think our study has gone on long enough to know whether sulindac has cardiovascular effects." There was an increase in cardio-vascular events, but it was not statistically significant.
The trial was launched only after two smaller "dose de-escalation" studies were done to identify the lowest effective dose of DFMO that could provide a clinical benefit. The dose used in this prevention trial is one-fiftieth of the dose used in the earlier treatment trials.
Based on earlier studies, some concerns do exist about DFMO-related toxicities, namely hearing loss. More patients in the DFMO/sulindac arm did experience minor reductions in hearing, as measured on audiograms. Participants who experienced hearing loss, mostly older participants, generally weren't aware of it.
"Across the entire group, the difference in hearing loss between treatment and placebo was approximately 2 decibels," he says. "That's [the sound of] rubbing your fingers together."
Dr. Meyskens' group is working with NCI to design larger phase III trials, including one in patients who have been treated for low-grade colorectal cancer. Currently, DFMO is not manufactured by any pharmaceutical company, so the team is also searching for industry partners to continue moving it through additional clinical trials and, they hope, regulatory approval.
"We hope these data will generate a lot of interest," Dr. Meyskens says.
—Carmen Phillips
|
 |
 |
Mutant Gene Linked to Aggressive Leukemia
Researchers have identified a common genetic change in Philadelphia chromosome (BCR-ABL1)-positive acute lymphoblastic leukemia (ALL), an aggressive leukemia that carries a poor prognosis. The change alters the gene IKZF1, which produces the protein Ikaros, and appears to be an important lesion in this ALL subtype, the researchers reported online in the April 13 Nature.
Ikaros plays a key role in regulating the normal development of lymphocytes. The IKZF1 gene alterations were detected as part of an ongoing effort to identify genetic abnormalities in ALL. Dr. James Downing of St. Jude Children's Research Hospital and his colleagues analyzed DNA copy number changes in 304 ALL cases, including 21 children and 22 adults with BCR-ABL1-positive disease.
 |
Looking Ahead: NCI Plan and Budget for FY 2009
An HTML version of The Nation’s Investment in Cancer Research: Connecting the Cancer Community - A Plan and Budget Proposal for Fiscal Year 2009 is now available at http://plan.cancer.gov. The annual plan and budget describes NCI’s strategies and progress to reduce the burden of cancer in the United States and includes requests for: an increase of $334 million to allow NCI to sustain current programs, restore some funding cuts, and provide for minimal growth; and an increase of $768 million to initiate new and expand existing initiatives.
Hard copies can be ordered via e-mail at cisocc@pop.nci.nih.gov, phone at 1-800-4-CANCER,
fax at 301-339-7968, or online
at www.cancer.gov. |
|
The IKZF1 gene was deleted in more than 80 percent of the BCR-ABL1 ALL cases. The deletions resulted in reduced expression of Ikaros or the expression of abnormal forms of the protein. "The discovery of this molecular lesion gives us important insights into the genetic pathways that are disrupted in BCR-ABL1 ALL," said Dr. Downing. "These results could ultimately lead to more effective treatments."
The researchers also analyzed 23 cases of chronic myelogenous leukemia (CML), which also involves BCR-ABL1 and is commonly treated with imatinib (Gleevec). Left untreated, CML may progress from an indolent stage to an acute leukemia known as blast crisis. The researchers observed Ikaros deletions at the progression of CML to lymphoid blast crisis, suggesting that Ikaros mutations help determine the behavior of BCR-ABL1 leukemia.
"BCR-ABL1 is the hallmark of CML, but this disease is complex," noted lead author Dr. Charles Mullighan of St. Jude. "Our results show that other genetic lesions are involved in the progression of CML to blast crisis, and in de novo BCR-ABL1 ALL. These additional lesions may be critical in the response of these diseases to treatment."
Chromosome Region Linked to Lung Cancer
A region of chromosome 15 may contain genetic risk factors for lung cancer, three research teams reported online this month in Nature and Nature Genetics. The results are from the first genome-wide association studies to attempt to identify the genetic component of a disease that is closely associated with a strong environmental cause. Yet researchers have long known that genetic factors play a role in lung cancer risk, and recent studies have implicated this region in the disease.
More work is needed to identify which DNA sequences in the region are responsible for the increased risk observed in the studies. The leading suspects are three genes that produce proteins on the cell surface that bind to nicotine, triggering a cascade of cellular changes, including some related to cancer. These proteins may contribute to cancer in the absence of exposure to nicotine.
The studies were led by Dr. Kari Stefansson of deCODE Genetics in Iceland; Dr. Paul Brennan of the International Agency for Research on Cancer in Lyon, France; and Dr. Christopher Amos of the University of Texas M.D. Anderson Cancer Center. The results provide strong evidence for a link between DNA variants on chromosome 15 and lung cancer, but the studies differ on whether the connection is direct or mediated through smoking behavior, according to an accompanying commentary in Nature.
Even larger studies with detailed information about smoking patterns and addictive behavior will be needed to discern whether "these discoveries relate to the risk of smoking, the risk of lung cancer, or the risk of both," write Drs. Stephen Chanock of NCI's Division of Cancer Epidemiology and Genetics and David Hunter of the Harvard School of Public Health.
IV Iron Effective in Treating Chemotherapy-Induced Anemia
Two studies in the April 1 Journal of Clinical Oncology (JCO) found that intravenous (IV) iron significantly improves hemoglobin levels in patients taking erythropoietin-stimulating agents (ESAs) for chemotherapy-induced anemia compared with ESAs alone or ESAs plus oral iron.
Anemia occurs in up to 75 percent of cancer patients who undergo chemotherapy or radiation treatment in clinical trials, and ESA therapy has been found to correct the blood condition in only 50 to 70 percent of patients.
In the first JCO study, 86 percent of patients receiving the ESA darbepoetin alpha (Aranesp) plus IV iron achieved either a hemoglobin level of at least 12 g/dL or an increase of at least 2 g/dL over their baseline hemoglobin level, compared with 73 percent of patients who received the ESA alone or ESAs with oral iron. Patients receiving IV iron responded more quickly to ESA therapy, achieving target hemoglobin levels in a median of 50 days compared with 64 days for patients not receiving IV iron.
In the second study, 76.7 percent of patients receiving darbepoetin alpha with IV iron achieved target hemoglobin levels, compared with 61.8 percent of patients receiving ESA therapy alone.
Last month, the U.S. Food and Drug Administration's Oncologic Drug Advisory Committee recommended substantially limiting the use of ESAs to treat anemia in cancer patients after clinical trials showed an increased mortality risk in patients using ESAs to achieve hemoglobin levels of 12 g/dL or higher.
However, in an editorial accompanying the JCO articles, Dr. Michael Auerbach of Georgetown University writes: "These two studies add unique and useful information to a rapidly growing body of data supporting the routine use of IV iron as an adjunct to ESA therapy in appropriately selected oncology patients."
|
 |
 |

At AACR, New Science and an Important Dialogue
Each annual meeting of the large U.S. and international cancer research organizations has its own unique aspects, its own atmosphere. Over the past several years, the American Association of Cancer Research (AACR) annual meeting has begun to feature more clinical and translational research, while maintaining its focus on the basic sciences. It is this "bench to bedside and back" approach that makes it such an important meeting.
At this year's meeting, some conference rooms have been overflowing with attendees wanting to hear pre-eminent researchers discuss their latest thinking on topics such as making rational decisions about combination therapies with targeted agents. Meanwhile, many press conferences have featured young investigators who, often for the first time, are being given an opportunity to present exciting new data from important research projects they have led.
This year's meeting has been notable for other reasons. In particular, data from some early- and late-phase clinical trials have generated tremendous optimism.
Dr. Daniel Von Hoff presented exciting data from a phase I, first-in-man study involving an experimental Hedgehog pathway inhibitor. The data suggest that this agent has the potential to treat the rare patient who develops metastatic basal cell carcinoma. This trial is also a tribute to the tremendous basic research that elucidated this signaling pathway's potentially important role in cancer.
On a personal level, this year's meeting was also noteworthy because I had the opportunity to participate in what outgoing AACR President Dr. William Hait described as an "experiment:" a session in which, after a brief presentation, a number of attendees asked me questions about some of the most prominent issues facing NCI and the broader cancer community. In particular, it was clear that there is tremendous concern about ensuring that young investigators get the support and opportunities they need to become successful cancer researchers.
This is clearly a very important issue, and it is among my top priorities. Indeed, every time I testify before congressional committees or meet with legislators and their staff, I underscore the impact five years of flat budgets have had on the pipeline of young investigators and that despite the high priority NCI places on training and supporting young investigators, there are limits to what we can do with our available resources.
There were questions about investigator-initiated versus program-directed grants and team science as well. In my reply, I emphasized NCI's priority to vigorously protect the unsolicited grant portfolio during budgetary stresses - and these grants are anticipated to grow with new resources from future budgets.
Nonetheless, our individual investigator grants have eroded not only in their success rates but also in their size. Current awards are inadequate to support the cost of today's science. In addition, I was able to mention changes in other areas of our research portfolio, especially in our larger programs, to reflect the changing research environment. For example, as a result of recommendations from the Translational Research Working Group, adjustments are being made to integrate the Specialized Programs of Research Excellence (SPORE) program into NCI's broader translational research program, enhance peer review, and more closely align individual SPORE funding to its priority score.
In discussing the ways in which NCI is improving the clinical trials process, I also stressed how important it is for the institute to create opportunities for connectivity between academia and industry and also between research progress and community cancer care. On the clinical trials front, for example, the Cancer Biomedical Informatics Grid will be critical for connecting researchers and providing new tools and resources that have the potential to improve the efficiency of the clinical trials process.
As this dialogue and the AACR annual meeting have shown, the dedication of those in the cancer community to reduce the cancer burden has grown stronger despite the challenges we currently face.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |
AACR Annual Meeting Coverage
APC 5-Year Results: Adenoma Recurrence Reduced, Cardiac Risks Clearer
The 5-year results of the Adenoma Prevention with Celecoxib (APC) trial indicate that 2 years after daily use of celecoxib (Celebrex) has ended, there continues to be a modest reduction in the recurrence of colorectal polyps.
Initially presented at the 2006 AACR annual meeting, the trial's results showed that compared with placebo, adenoma recurrence and advanced adenoma recurrence were significantly reduced in participants who took celecoxib daily for 3 years. Participants, all of whom had previously had lesions removed, had significantly fewer total adenomas and advanced adenomas at 3 years.
Speaking yesterday at the 2008 AACR annual meeting in San Diego, CA, the trial's lead investigator, Dr. Monica Bertagnolli, reported that although the effect diminished after the drug was discontinued, a treatment benefit was still present at 5 years. Celecoxib use, however, was associated with an increased risk of serious side effects, including heart attacks and strokes. The safety analysis suggests that these serious adverse events were greatest in participants with pre-existing cardiovascular risk factors.
Overall, compared with participants in the placebo arm, advanced adenoma risk was reduced by 41 percent among patients taking the 400 mg/day dose and 26 percent among patients taking the 800 mg/day dose.
Cardiovascular events were far more likely among participants who had at least two pre-existing cardiovascular risk factors (e.g., high blood pressure, diabetes, age of more than 65 years) at study entry: 5.9 percent of participants in the placebo arm, compared with 8.2 percent in the 400 mg/day arm and 11.2 percent in the 800 mg/day arm, reported a cardiovascular adverse event. By contrast, for APC participants who had no cardiovascular risk factors at study entry, the rates of cardiovascular adverse events were 0.9 percent, 3.9 percent, and 1.9 percent, respectively.
The results, said Dr. Bertagnolli, of Brigham and Women's Hospital demonstrate that COX-2 inhibitors "are risky for some patients." But, she continued: "Our study also shows that for patients without major cardiovascular risk factors, celecoxib at low doses protects against high-risk lesions that can lead to colon cancer."
These results come on the heels of a more extensive, NCI-funded cross-study safety analysis of six placebo-controlled studies involving celecoxib. These data were presented in late March at the annual meeting of the American College of Cardiology. That analysis showed a threefold increased risk of cardiovascular events in patients given the highest celecoxib dose, but also showed that risk was significantly higher among study participants with underlying cardiovascular risk factors.
Finding and Studying Pancreatic Cancer Stem Cells
A refined strategy for isolating cancer stem cells in pancreatic tumors and preliminary evidence that these rare cells may influence patient survival were presented at the 2008 AACR annual meeting. The results are from a series of studies at the Johns Hopkins Kimmel Cancer Center aimed at understanding the potential role of pancreatic cancer stem cells in this deadly disease.
The cancer stem cell hypothesis holds that many cancers are initiated and driven by these elusive cells, which are capable of perpetuating themselves while giving rise to tumors.
In 2003, lead investigator Dr. William Matsui and his colleagues identified cancer stem cells in patients with multiple myeloma, a cancer of the bone marrow. Building on this work, the researchers have now developed a strategy for isolating potential pancreatic cancer stem cells that integrates markers from different studies.
The strategy combines the surface proteins CD44 and CD24 (used last year by a University of Michigan team to identify pancreatic cancer stem cells) with the enzyme aldehyde dehydrogenase, which was a marker in the Hopkins multiple myeloma studies.
Using all three markers together yielded a stem cell population that was 2 to 10 times more concentrated than using either type of marker alone, Dr. Zeshaan Rasheed from Johns Hopkins reported at AACR.
In further studies, the researchers found that patients whose tumors lack the cancer stem cell markers live a few months longer than other patients. This difference may be meaningful in a disease in which most patients die within a year of diagnosis.
"We're starting to get some inkling that these cells are clinically relevant in this disease," noted Dr. Matsui. His team has been investigating whether cancer stem cells contribute to metastasis, and a publication describing all of the studies is planned.
Synthetic Vitamin D Shows Anti-cancer Effect and No Toxicity in Mice and Rats
Researchers at Rutgers University have developed a form of active vitamin D called Gemini 0097 that dramatically reduced the growth of both ER-positive and ER-negative breast cancer cells and showed no toxicity in rats and mice. Dr. Nanjoo Suh presented the results of her team's study at AACR yesterday.
The active form of vitamin D is a hormone synthesized by the skin, liver, and kidneys from dietary precursors. Epidemiological studies have shown that people deficient in active vitamin D have a higher risk of colorectal, breast, prostate, and other cancers.
Clinical studies using vitamin D supplements to prevent cancer have produced mixed results, in part because of the fact that high doses of vitamin D in its active form can cause an imbalance in other electrolytes, including toxic levels of calcium in the blood. For this reason, researchers have been testing analogs of vitamin D, chemically modified forms of the vitamin that have a slightly different molecular structure.
The Rutgers team tested 60 new analogs of active vitamin D against a placebo in rats that had been exposed to mammary carcinogens. One of the analogs, Gemini 0097, was superior for preventing tumor development, cutting ER-positive breast cancer growth by 60 percent. In a mouse model of ER-negative breast cancer, Gemini 0097 reduced tumor development by 50 percent. Blood calcium levels remained normal in mice and rats that received the analog treatment.
"Our analog has a side-chain that makes the molecule much bulkier than naturally occurring vitamin D, such that the binding of the molecule to its receptor is only 40 percent, but somehow the activity is better," explained Dr. Suh. She speculated that this superior activity may be caused by alternative recruitment of co-factors to the vitamin D receptor. Dr. Suh noted that more data from animal studies are needed before Gemini 0097 is tested in humans.
Reduction in Breast Cancer Incidence Differs by Race
Recent studies have reported that after rising for two decades, the breast cancer incidence rate in the United States dropped sharply between 2002 and 2003 and continued to decline in 2004. However, new research presented April 15 at the 2008 AACR annual meeting shows that this decline was not equally distributed across racial groups.
Investigators from the University of Chicago used data from 17 cancer registries of the Surveillance, Epidemiology, and End Results program to calculate changes in incidence for invasive and in situ female breast cancer between 2000 and 2004 by race and tumor stage in women aged 50 to 69.
The incidence rate of in situ cancer remained stable in most groups of women. However, the investigators found that while rates of invasive breast cancer decreased significantly for Caucasian women by the end of 2003, the incidence rates did not change significantly for African American, American Indian/Alaskan Native, or Asian American/Pacific Islander women.
"This racial disparity is consistent with the hypothesis that discontinuation of hormone replacement therapy (HRT) may have caused the dramatic reduction," stated the authors in their abstract presented at AACR.
"We suspect that the widespread discontinuation of menopausal hormone use had a greater effect on Caucasians," said Dr. Dezheng Huo in a press release from the University of Chicago.
African Americans are less likely to develop breast cancers that are receptive to estrogen, Dr. Huo explained, so they were harmed less by taking hormones and benefited less by discontinuing them.
In addition, African Americans had "a similar magnitude of reduction in hormone therapy," said Dr. Huo, "yet it did not lead to any benefit, suggesting that genetic variations in estrogen and progesterone metabolism play a role."
| |
 |
 |

Improving Unrelated Hematopoietic Stem Cell Transplants
The field of hematopoietic stem cell transplantation (HSCT), in which cancer patients and people with certain other diseases receive transplanted blood-producing stem cells to restore their blood and immune systems after treatment, has seen several exciting developments in recent years.
The discovery of non-bone marrow stem cell sources, including peripheral blood and fetal cord blood, has increased the supply of unrelated-donor (allogeneic) stem cells available for patients. And lower-toxicity treatment regimens - mini-transplants that merely suppress a patient's immune system before the transplant, rather than eliminate all blood-forming cells - have allowed patients who would previously have been ineligible for the procedure to receive it, including older adults and those who have comorbid conditions.
Hoping to build on these previous successes, researchers in the NCI Center for Cancer Research's Experimental Transplantation and Immunology Branch (ETIB) have launched a new initiative to explore how to optimize HSCT to maximize the benefits of the procedure while minimizing its risks.
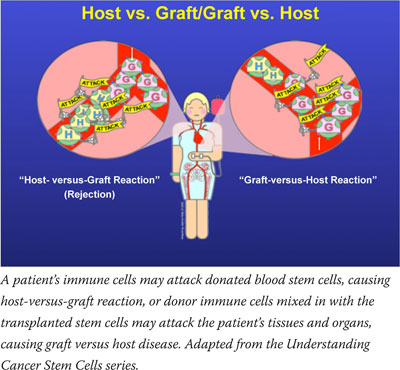 "Our goal is to overcome the four primary barriers to HSCT: rejection, graft-versus-host-disease, tumor relapse, and lack of immune reconstitution," explains ETIB Chief Dr. Ronald Gress. The factor that influences each of these barriers most is antigen mismatching between an HSCT donor and recipient. Human leukocyte antigens are important proteins for determining this match, but other, lesser-known cell-surface proteins also play a role.
"Our goal is to overcome the four primary barriers to HSCT: rejection, graft-versus-host-disease, tumor relapse, and lack of immune reconstitution," explains ETIB Chief Dr. Ronald Gress. The factor that influences each of these barriers most is antigen mismatching between an HSCT donor and recipient. Human leukocyte antigens are important proteins for determining this match, but other, lesser-known cell-surface proteins also play a role.
When a match is imperfect, transplanted cells can react against the recipient in what is called graft-versus-host disease, causing damage throughout the body and possible death. However, mismatched transplanted immune cells are also capable of doing what the recipient's immune system could not: recognizing and killing cancer cells. This reaction is called graft-versus-tumor effect, and with some forms of cancer, it not only restores the immune system, but actually cures the disease.
A part of this new ETIB initiative is a pilot study to look at HSCT from fully matched unrelated allogeneic donors and its potential to cure. Senior Investigator Dr. Michael Bishop and colleagues will compare two treatments that have been used in preventing graft-versus-host disease after mini-transplants: a combination of tacrolimus, methotrexate, and sirolimus; and a combination of cyclosporine and alemtuzumab.
The primary objective of the pilot study is to look at the safety of each regimen, as well as how each affects the speed of engraftment and immune reconstitution after the transplant. A secondary objective is to examine immune reconstitution using a technique known as CDR3 spectratyping, where polymerase chain reaction and gel electrophoresis are used to analyze the diversity and population of T cells against antigens.
"We're taking a personalized approach to transplantation," says Dr. Bishop. In the early days, he explains, all patients received the same transplant preparation drug doses, regardless of what treatments they had previously. "This is like using a really big hammer for every size nail," he says.
But how intact a patient's immune system is varies according to how much chemotherapy the person has received. Patients who have very weak immune systems do not need the same doses of immune-suppressing drugs as someone whose immune system is comparatively strong. T-cell number revealed by CDR3 spectratyping can guide this dosing decision, determining the competence of the rejection response in these patients.
"We can use this personalized preparation, which was developed with Dr. Dan Fowler in ETIB, to lower the immune systems only as much as is needed, so that each patient is on an equal playing field and each person has an equal chance that their graft will be successful, while sparing patients unnecessary toxicity," explains Dr. Bishop. The spectratyping can be used afterward to show how well each patient's immune system recovers.
Dr. Bishop's pilot study marks the first time that allogeneic transplants from unrelated donors are being studied by NCI at the NIH Clinical Research Center (CRC), and it will establish a platform in ETIB for future studies, including the use of cytokine-defined T-cell subsets to reduce transplant rejection and graft-versus-host disease, ways to increase function of the thymus gland to improve immune reconstitution, and new strategies to prevent and treat disease relapse.
"Unrelated transplants are being performed all over the country, but the ability to engraft cells from an unrelated donor while minimizing chemotherapy and maximizing graft-versus-tumor effect is not being tested anywhere else in this individualized way," says Dr. Gress, who notes that several other institutes at NIH have a stake in the HSCT research being performed.
—Brittany Moya del Pino
|
 |
 |

Herbal Therapy for Brain Cancer
Name of the Trial
Phase II Randomized Study of Adjuvant Boswellia Serrata and Standard Treatment Versus Standard Treatment Alone in Patients with Newly Diagnosed or Recurrent High-Grade Gliomas (CASE-CCF-7348). See the protocol summary at http://www.cancer.gov/clinicaltrials/CASE-CCF-7348.
 Principal Investigator
Principal Investigator
Dr. Glen Stevens, Cleveland Clinic Foundation
Why This Trial Is Important
High-grade gliomas are among the most common and aggressive forms of adult brain cancer. Swelling of the brain (brain edema) is an often debilitating symptom of glioma and may continue to affect patients even if the tumor is surgically removed.
Resin from the Boswellia serrata tree (frankincense) has been shown in animal and human studies to reduce inflammation, which is a primary cause of brain edema. Additionally, laboratory studies suggest that B. serrata resin may also cause human brain cancer cells to undergo programmed cell death (apoptosis).
In this trial, patients will be randomly assigned to take an herbal preparation of B. serrata orally four times a day in conjunction with standard treatment for six months or to take standard treatment alone for six months. All patients are advised to eat a low-fat healthy diet. Diets rich in red meat contain a substance called arachidonic acid, and chemicals in fat can be converted to arachidonic acid. Arachidonic acid is converted in the brain to signaling molecules called eicosanoids that may promote inflammation and tumor growth. Doctors want to see if B. serrata can help reduce brain edema, tumor growth, and levels of 5-lipoxygenase, an enzyme that helps convert arachidonic acid to eicosanoids, in these patients when combined with standard treatment.
"Some small studies have suggested that frankincense extract may help limit brain edema and even have an anti-tumor effect,” said Dr. Stevens. "We hope that use of this herbal preparation in conjunction with a healthy diet will help improve patient outcomes and act in a complementary fashion with standard treatments for high-grade gliomas."
For More Information
See the lists of entry criteria and trial contact information at http://cancer.gov/clinicaltrials/CASE-CCF-7348 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
 AMA Honors NCI's Linehan
AMA Honors NCI's Linehan
Dr. W. Marston Linehan, head of the Urologic Oncology Branch in NCI's Center for Cancer Research, has received the American Medical Association's (AMA) Nathan Davis Award for Outstanding Government Service.
Dr. Linehan received the award at the AMA's national advocacy conference on April 1 in recognition for his leadership and contributions to kidney cancer research. Dr. Linehan and his colleagues have discovered multiple genes linked to kidney cancer and established a comprehensive kidney cancer program at NCI to study families with the disease.
The Nathan Davis Award, named for the founding father of the AMA, recognizes elected and career officials in federal, state, or municipal service whose contributions have promoted the art of science or medicine and the betterment of public health.
 Hoover Receives AACR Award
Hoover Receives AACR Award
Dr. Robert N. Hoover, director of the Epidemiology and Biostatistics Program in NCI's Division of Cancer Epidemiology and Genetics, received the American Association for Cancer Research (AACR)-American Cancer Society Award for Research Excellence in Cancer Epidemiology and Prevention for his pioneering research in identifying environmental and genetic determinants of cancer, most notably bladder and breast cancer. Dr. Hoover delivered the award lecture today at the AACR annual meeting in San Diego, CA. The AACR and the American Cancer Society established this award in 1992 to honor outstanding research accomplishments in the fields of cancer epidemiology, biomarkers, and prevention.
 Monograph on Middle East Palliative Care Available
Monograph on Middle East Palliative Care Available
NCI's Office of International Affairs and the Middle East Cancer Consortium (MECC) recently released Palliative Care in the Region Represented by the Middle East Cancer Consortium. The monograph is a review and comparative analysis of the state of palliative care in Cyprus, Egypt, Jordan, Israel, Turkey, and the Palestinian Authority. NCI, a major sponsor of MECC, commissioned the International Observatory on End-of-Life Care to conduct the study. The monograph can be found at http://oia.cancer.gov/pdf/pcmonograph.pdf. For more information, contact Dr. Joe Harford at harfordj@mail.nih.gov.
 NCI Cancer Bulletin Wins Award
NCI Cancer Bulletin Wins Award
The NCI Cancer Bulletin won a silver award in the miscellaneous/professional category, the highest recognition in that category this year, in the Fall/Winter 2007 World Wide Web Health Awards competition. The awards competition recognizes the best Web-based, health-related content for consumers and professionals. Nearly 1,000 submissions were received from hundreds of public and private health-related organizations.
| |
 |
 |
Following are newly released NCI research funding opportunities:
Cancer Education Grants Program
Announcement Number: PAR-08-120
Application Receipt Dates: Non-AIDS Applications (new, renewal, resubmission, or revision):
May 25 and Sept. 25, 2008; Jan. 25, May 25, and Sept. 25, 2009; Jan. 25, May 25, and Sept. 25, 2010; Jan.25, and May 25, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, and Sept. 7, 2011.
This is a renewal of PAR-06-540 and will use the R25 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3865. Inquiries: Dr. Lester S. Gorelic - gorelicl@mail.nih.gov.
Symptom Interactions in Cancer and Immune Disorders
Announcement Number: PA-08-121
Application Receipt Dates: Non-AIDS Applications (new): June 5 and Oct. 5, 2008; Feb. 5, June 5, and Oct. 5, 2009; Feb. 5, June 5, and Oct. 5, 2010; Feb. 5, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This is a renewal of PA-07-009 and will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3866. Inquiries: Dr. Ann M. O'Mara - omaraa@mail.nih.gov.
Enhancing Tumoricidal Activity of Natural Killer Cells by Dietary Components for Cancer Prevention
Announcement Number: PA-08-131
Application Receipt Dates: Non-AIDS Applications (new): June 5 and Oct. 5, 2008; Feb. 5, June 5, and Oct. 5, 2009; Feb. 5, June 5, and Oct. 5, 2010; Feb. 5, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This funding opportunity will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3867. Inquiries: Dr. Young S. Kim - yk47s@nih.gov.
Correlative Studies with Specimens from Multisite Trials
Announcement Number: PA-08-134
Application Receipt Dates: Non-AIDS Applications (new): June 5 and Oct. 5, 2008; Feb. 5, June 5, and Oct. 5, 2009; Feb. 5, June 5, and Oct. 5, 2010; Feb. 5, June 5, and Oct. 5, 2011; Feb. 5, 2012.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, and Sept. 7, 2011; Jan. 7, and May 7, 2012.
This is a renewal of PA-07-177 and will use the R01 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3868. Inquiries: Dr. Heng Xie - xiehe@mail.nih.gov.
Symptom Interactions in Cancer and Immune Disorders
Announcement Number: PA-08-122
Application Receipt Dates: Non-AIDS Applications (new): June 16 and Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This is a renewal of PA-07-009 and will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3866. Inquiries: Dr. Ann M. O'Mara - omaraa@mail.nih.gov.
Enhancing Tumoricidal Activity of Natural Killer Cells by Dietary Components for Cancer Prevention
Announcement Number: PA-08-132
Application Receipt Dates: Non-AIDS Applications (new): June 16 and Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This funding opportunity will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3867. Inquiries: Dr. Young S. Kim - yk47s@nih.gov.
Correlative Studies with Specimens from Multisite Trials
Announcement Number: PA-08-133
Application Receipt Dates: Non-AIDS Applications (new): June 16 and Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): May 7 and Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This is a renewal of PA-06-296 and will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3868. Inquiries: Dr. Heng Xie - xiehe@mail.nih.gov.
Exploratory/Developmental Grant for Complementary and Alternative Medicine Studies of Humans
Announcement Number: PAR-08-135
Application Receipt Dates: Non-AIDS Applications (new): June 16 and Oct. 16, 2008; Feb. 16, June 16, and Oct. 16, 2009; Feb. 16, June 16, and Oct. 16, 2010; Feb. 16, 2011.
AIDS and AIDS-Related Applications (new, renewal, resubmission, or revision): Sept. 7, 2008; Jan. 7, May 7, and Sept. 7, 2009; Jan. 7, May 7, and Sept. 7, 2010; Jan. 7, May 7, 2011.
This is a renewal of PA-06-510 and will use the R21 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3869. Inquiries: Dr. Jeffrey White - jeffreyw@mail.nih.gov.
|
 |
 |
A Conversation with Dr. Asad Umar
Dr. Asad Umar, acting chief of the Gastrointestinal and Other Cancers Research Group in NCI's Division of Cancer Prevention, offers his insights into the implications of two major colorectal cancer chemoprevention studies presented at the 2008 AACR annual meeting.
What is your reaction to the APC and DFMO/sulindac trial results?
NCI's Division of Cancer Prevention funded the DFMO/sulindac study, and we were confident that this was a well-designed study. We now see that this is an excellent combination for preventing the recurrence of colorectal adenomas, particularly advanced adenomas, which have the highest risk of becoming cancer.
The big question is always: How will the animal model results translate to human trials? In this case, the animal models predicted accurately. Nonsteroidal anti-inflammatory drugs like celecoxib and sulindac, and the ornithine decarboxylase inhibitor DFMO, have all shown efficacy as single agents, and there was a synergistic effect when they were given in combinations in animal chemoprevention studies.
In the APC trial, the efficacy did drop after participants stopped taking the drug, but there was still a statistically significant reduction in adenomas after being off the drug for two years. And, importantly, the strongest effect was seen in advanced adenomas, the polyps most likely to progress into cancer.
What are your thoughts about the cardiac affects related to celecoxib in the APC trial?
The NCI-funded meta-safety analysis by Dr. [Scott D.] Solomon and colleagues, which includes the APC trial, provides a better understanding of celecoxib's safety profile. By pooling the safety data from six different trials, we can better assess the cardiovascular risk of celecoxib.
We saw that this risk was greatest in those with pre-existing cardiac risk factors. In those patients without pre-existing risk factors at baseline, there was no significant risk of a cardiovascular adverse event, even with a high celecoxib dose. This result should be interpreted with some caution because the number of individuals in the low cardiac-risk category was small. However, this is an important finding because we can stratify patients according to their underlying cardiac risk factors in future studies.
We now have two approaches that may effectively prevent colorectal cancer precursors. What are the next steps?
There are a couple of things that we need to think about. How do we best design the next celecoxib trials to stratify populations into appropriate risk categories and minimize the risk of cardiovascular toxicity? We need to identify the population in which the benefits of polyp prevention will be greater than any potential risk of taking celecoxib.
We also need to better define the lowest effective dose and dosing regimen. Participants in the PreSAP trial, who were given 400 mg of celecoxib once a day, experienced fewer instances of cardiovascular toxicity compared to patients who took 200 mg twice a day or 400 mg twice a day. We can design the next trial to evaluate a once-a-day regimen with a lower dose or an every-other-day regimen. We also might want to conduct additional animal studies to see if we can decrease toxicity and maintain efficacy for adenoma prevention with a "drug holiday" approach.
As for DFMO/sulindac, Dr. [Frank] Meyskens and his colleagues are planning a larger trial of this combination. To get regulatory approval, we'll need to show that these drugs, as single agents, are effective. Future trials will need to include several treatment arms: sulindac alone, DFMO alone, and DFMO plus sulindac.
We are also excited that, based on the results from these studies, other investigators are interested in studying the mechanisms of chemoprevention for nonsteroidal anti-inflammatory drugs and DFMO, and we have requests to test DFMO as a cancer prevention agent in other settings including skin cancer. These agents may well have applicability beyond colorectal cancer. |
 |
|