FY 2009 Trans-NIH AIDS Research By-Pass Budget Estimate
The HIV/AIDS Pandemic
Worldwide1
- 33.2 million people estimated to be currently living with HIV/AIDS infection.
- More than 25 million men, women and children have already died.
- In 2007, an estimated 2.5 million new HIV infections occurred worldwide.
- In 2007, 2.1 million people died from AIDS.
In the United States2
- Approximately 1 million people are living with HIV/AIDS.
- In 2005, an estimated 40,000 people were newly infected with HIV, and 16,300 died from AIDS.
- Since 1981, 953,000 people have been diagnosed with AIDS, of whom 530,000 have died.
- Racial and ethnic populations are disproportionately affected HIV/AIDS.
1UNAIDS. AIDS Epidemic Update, 2007.
2CDC. HIV/AIDS Surveillance Report, 2005. Vol. 1717. Rev. ed.
Contents
- Legislative Mandate
- Introduction
- OAR Mission
- The NIH AIDS Research Program
- OAR By-Pass Budget Estimate
- Annual Trans-NIH AIDS Research Budget Linked to Strategic Plan
- Trans-NIH AIDS Research Priorities for FY 2009
- NIH Research to Address these Priorities:
- List of Supporting Documents
- Table: Funding by the Trans-NIH Plan for HIV-Related Research
- Table: Funding by Research Mechanism
Legislative Mandate
Public Law 103-43, the National Institutes of Health Revitalization Act of 1993, requires that “the Director of the Office of AIDS Research establish a comprehensive plan for the conduct and support of all AIDS activities of the agencies of the National Institutes of Health.” It also requires that the Director “shall prepare and submit directly to the President, for review and transmittal to the Congress, a budget estimate for carrying out the Plan for the fiscal year....” That budget “shall estimate the amounts necessary for the agencies of the National Institutes of Health to carry out all AIDS activities determined by the Director of the Office to be appropriate, without regard to the probability that such amounts will be appropriated.”
Introduction
In accordance with the law, the Office of AIDS Research (OAR) has developed the fiscal year (FY) 2009 NIH AIDS Research Professional Judgment (By-Pass) Budget Estimate to carry out the scientific priorities of the FY 2009 Trans-NIH Plan for HIV-Related Research. This budget estimate is based on the commitment to support the highest quality research and the urgent need to pursue priority scientific opportunities.
This By-Pass budget request: addresses critical new scientific needs; addresses gaps in our understanding through a renewed emphasis on basic science; begins to restore vital resources that have been sapped by the effects of inflation; and capitalizes on emerging scientific opportunities by providing additional funds for new, exciting areas of investigation, including a new program to utilize genomics tools to investigate the immune response to HIV infection.
OAR Mission
The OAR coordinates the scientific, budgetary, legislative, and policy elements of all NIH AIDS research. OAR, located within the NIH Office of the Director, was authorized to plan, coordinate, and evaluate all AIDS research conducted or supported by NIH. The OAR has utilized these authorities to establish unique trans-NIH planning, budgeting, and portfolio assessment processes. These processes promote collaboration, minimize duplication, and ensure that NIH AIDS research dollars are invested in high priority research that ultimately will lead to the development of new tools for use in the global fight against AIDS.
The NIH AIDS Research Program
NIH has established the largest and most significant AIDS research program in the world. NIH supports and conducts a comprehensive program of basic, clinical, and behavioral research on HIV infection and its associated co-infections, opportunistic infections, malignancies, and other complications. Perhaps no other disease so thoroughly transcends every area of clinical medicine and basic scientific investigation, crossing the boundaries of the NIH Institutes and Centers (ICs).
NIH-funded research has lead to the discovery of antiretroviral therapies and regimens that have resulted in improved quality of life and life expectancy for those with access to these drugs. In addition, NIH research has led to the development of treatments for some HIV-associated co-infections and co-morbidities, including malignancies, neurological complications, tuberculosis, and other clinical manifestations. NIH research also has led to a number of advances in HIV prevention, including strategies for the prevention of mother-to-child transmission and the demonstration that medically supervised circumcision of adult men can reduce risk of heterosexual HIV acquisition.
Despite these important advances, the epidemic continues to expand, and improved prevention strategies and therapeutic regimens are critically necessary. The AIDS pandemic will continue to wreak devastating consequences in the United States and around the world for decades to come. The pandemic affects the future of families, communities, military preparedness, national security, political stability, national economic growth, agriculture, business, health care, child development, and education in countries around the globe.
OAR By-Pass Budget Estimate
The FY 2009 By-Pass budget request for NIH AIDS research is $3.35 billion, which represents a 15 percent increase over the FY 2008 Enacted level. The 15 percent increase requested in this By-Pass budget represents an initial investment – a down payment -- that must be maintained and enhanced to begin to address the impact of the erosion of buying power on critical research programs, to restore lost opportunity, and to take advantage of emerging scientific advances. This amount includes the total trans-NIH support for intramural and extramural research; research management support; research centers; and basic and clinical research on HIV/AIDS, as well as the wide spectrum of AIDS-associated malignancies, opportunistic infections, co-infections, and clinical complications.
This request takes into account the serious impact of inflation, as measured by the Biomedical Research and Development Price Index (BRDPI), and the flattening of the NIH AIDS budget over the past five years on NIH buying power. Together these factors have effectively reversed the doubling of the budget that occurred between the fiscal years 1998 and 2003. The following chart demonstrates the impact of those factors on the buying power of the NIH AIDS research program.
FY 2003–FY 2009 Current and Constant Projections: NIH HIV/AIDS Research Dollars (dollars in thousands)
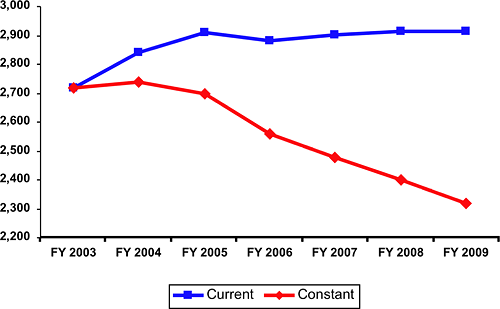
In addition to inflation, the decline of the U.S. dollar has further eroded buying power for AIDS researchers conducting studies in international settings. AIDS represents the largest proportion of the NIH international research portfolio. As the dollar has declined against the currencies in many of these countries where AIDS research is ongoing, the value or purchasing power of these international awards has diminished over the past years.
It is important to note that these budgetary constraints have occurred during a time of significant public health need and scientific opportunity. Increased demand and urgency for government funding is further necessitated by concurrent significant reductions in pharmaceutical and biotechnology company investment in basic and clinical AIDS biomedical research.
Annual Trans-NIH AIDS Research Budget Linked to Strategic Plan
OAR manages and coordinates the multifaceted and complex NIH AIDS research agenda. Each year, the OAR develops the Trans-NIH Plan for HIV-Related Research to ensure the AIDS budget is used to fund the highest priority AIDS-related research. The FY 2009 Trans-NIH Plan for HIV-Related Research can be accessed on the OAR website at http://www.oar.nih.gov/strategicplan/fy2009/. The Plan provides the framework for formulation of the annual trans-NIH AIDS research budget. The budget process continues at each step of the budget development process through to the final Congressional appropriation. Dollars are allocated to ICs based not on a formula, but on the priorities of the Plan, scientific opportunities, and the capacity of individual ICs to absorb and expend resources for the most meritorious science. This By-Pass budget request reflects the proposed initiatives requested by the ICs to support their critical ongoing research as well as new priority initiatives.
Trans-NIH AIDS Research Priorities for FY 2009
The FY 2009 Trans-NIH Plan for HIV-Related Research identified two critical priorities that transcend all areas of AIDS research. These over-arching priorities shaped the development of this budget request:
- Prevention of acquisition and transmission of HIV: Prevention of HIV infection is NIH’s highest priority for AIDS-related research. The NIH prevention research agenda includes basic, translational, and clinical research on microbicides and vaccines development; and behavioral and social sciences associated with HIV transmission and acquisition. There is an urgent need to expand the range of interventions for preventing HIV transmission beyond those currently available as new HIV infections continue at an unacceptably high rate globally, including in the United States. Studies of novel prevention strategies, such as circumcision to prevent heterosexual HIV acquisition in men, pre-exposure uses of antiretroviral therapy to prevent HIV infection, and strategies that can be used in resource-limited settings, must be continued.
- Prevention and treatment of HIV-associated co-morbidities, co-mortalities, and co-infections: Recent epidemiologic studies and clinical reports show an increased incidence of HIV-associated co-morbidities, co-mortalities, and co-infections, including cardiovascular complications, malignancies, neurological complications, tuberculosis, and other clinical manifestations, associated with long-term HIV disease and prolonged antiretroviral therapy. Research that will lead to a better understanding of these HIV-associated conditions is a high priority for the NIH, including research on how antiretroviral drugs may cause these manifestations and complications and the complex pathogenesis associated with HIV co-infections. In addition, translational and clinical studies are needed to transform fundamental research results into improved strategies for preventing and treating these HIV-associated co-morbidities, co-mortalities, and co-infections.
NIH Research to Address These Priorities:
The FY 2009 NIH By-Pass budget request for HIV/AIDS research responds to these critical priorities in the following key areas:
Microbicides
This FY 2009 By-Pass budget places high priority on microbicide research, requesting $128 million in this area, representing a $25 million (24.3 percent) increase over the FY 2008 Enacted level.
Around the world, most HIV infections are spread through heterosexual transmission; and half of all infected adults are women. Women have no means to protect themselves from HIV if their partners do not use a condom or allow a female condom to be used. Prevention methods such as abstinence or being faithful are not likely to protect married women or those who are sexually abused. A safe and effective microbicide would provide women a means to protect themselves from HIV.
NIH supports a comprehensive microbicide research program that includes the screening, discovery, development, preclinical testing, and clinical evaluation of microbicide candidates, as well as fundamental research aimed at understanding how HIV transverses mucosal membranes and infects cells. In addition, NIH supports behavioral and social science research on the acceptability and use of microbicides among different populations. NIH has undertaken critical efforts over the years to attract investigators into this field.
NIH has initiated a series of administrative steps to increase the level of awareness and focus on microbicide research, including: the establishment of a Microbicide Research Working Group, comprised of non-government experts who will play a unique and critical role in guiding the formulation of the NIH microbicide agenda; establishment of a new microbicide research branch at NIAID; and the Microbicide Innovation Program, designed to accelerate the discovery and development of single and/or combination microbicides.
Recent clinical trial results of microbicide candidates have been disappointing, and demonstrate the need for renewed and intensified efforts to fund additional research to understand basic science questions and develop new approaches to designing microbicides.
Budget Policy
NIH is unable to adequately address this important area of investigation without additional funds above the FY 2008 Enacted level. Therefore, this By-Pass budget requests increased support for basic science initiatives on the mechanisms to interrupt HIV transmission.
It is critical for NIH to increase collaborations with academia, industry, and foundations to identify and explore new and existing compounds as potential topical microbicidal agents. Without this By-Pass Budget request level, NIH will be unable to: provide adequate funds to support the evaluation of novel lead candidates in animal models with unique mechanisms of action; expand the initiative for development of new innovative microbicide concepts; or accelerate the integrated pre-clinical/clinical program for development of lead microbicide candidates. This By-Pass Budget also requests additional funds for the development of standardized criteria for selecting potential products for evaluation in clinical trials and for advancing them through the different phases of preclinical and clinical studies. Additional funds are requested to provide essential support for the Microbicide Trials Network and the infrastructure necessary to conduct microbicide trials, especially in developing countries, as well as to fund important research on ethical and behavioral issues impacting these clinical trials.
Vaccines
The best long-term hope for controlling the AIDS pandemic is the development of safe, effective, and affordable HIV vaccines. AIDS vaccine research remains a high priority to ensure that new and innovative concepts continue to advance through the pipeline. NIH supports a broad HIV vaccine research portfolio encompassing basic, preclinical, and clinical research. This By-Pass budget requests $681 million for this area, an increase of $88 million (14.8 percent) over the FY 2008 Enacted level.
In FY 2007, NIH supported a number of Phase I, II, and IIb clinical trials. Two large studies conducted by NIH in partnership with Merck & Co., Inc. were halted by the Data and Safety Monitoring Board after interim analyses of data because the vaccine candidate did not prevent HIV infection. Although disappointing, the results from these clinical studies underscore the critical need to invest in basic research on the virus and host immune responses that can inform the development of new and innovative vaccine concepts; as well as the development of improved animal models to conduct pre-clinical evaluations of vaccine candidates. Due to the similarities of the Merck vaccine candidate with the candidates developed by the NIH Vaccine Research Center (VRC), the clinical protocol design for the planned VRC PAVE 100 clinical trial will require extensive modification and additional monitoring and testing of study volunteers, adding additional cost to this large NIH-sponsored study. Without additional funds, the study will be severely impacted.
Budget Policy
This By-Pass budget requests additional funds above the President’s request to support basic research studies in vaccine development. One such important study is ongoing at the NIH Dale and Betty Bumpers Vaccine Research Center (VRC). NIH intramural scientists at the VRC determined the long-sought picture of the precise interaction of the HIV surface protein gp120 as it looks when bound to an infection-fighting antibody, a finding that could have profound implications for HIV vaccine design. In addition, researchers at the NIH-sponsored Center for HIV/AIDS Vaccine Immunology (CHAVI) recently reported significant findings from genomics experiments comparing the genome of long-term non-progressors to those who experienced rapid disease progression.
These results highlight the urgent need for an increased emphasis on genomic studies of the human immune system. Without the additional funds requested in this By-Pass budget, NIH will be unable to fund additional basic research on HIV and host immune responses. Findings from this important research could provide new information for the design and development of new vaccine concepts and the pre-clinical/clinical development of vaccine candidates in the pipeline. These funds are critically needed to support these changing priorities in HIV vaccine research.
Behavioral and Social Science
Behavioral and social science research are essential components of the NIH prevention science agenda. This By-Pass budget requests $478 million in this area, an increase of $60 million (14.4 percent) over the FY 2008 Enacted level.
NIH supports research to further our understanding of how to change the behaviors that lead to HIV acquisition, transmission, and disease progression—including preventing their initiation—and how to maintain protective behaviors once they are adopted. In addition, NIH supports research aimed at better understanding the social and cultural factors associated with HIV risk or protection, particularly in communities at high risk of HIV acquisition. This research will contribute to the implementation of a broader range of preventive and/or therapeutic strategies.
Behavioral issues associated with adherence to therapies are another area of priority investigation. Lack of complete adherence to drug regimens may result in the development of drug-resistant strains of HIV, which could have devastating public health implications. In addition, HIV-infected individuals taking antiretroviral therapies who experience improved health and a decline in detectable virus may believe that they are less infectious and may lapse into unsafe sexual and drug-using behaviors. This could have the effect of increasing HIV transmission, if the virus is still viable at undetectable levels.
Budget Policy
This By-Pass budget requests additional support for expansion of ongoing research to develop and test effective HIV-related interventions that build on studies of substance addiction and the complex interaction of alcohol use, drug use, and disinhibition. This By-Pass budget also requests additional funds that would permit NIH to support new global partnership initiatives for social science research on AIDS and studies on the role of behavioral and social networks in HIV transmission. Without these additional funds, NIH will be unable to support the development and evaluation of effective interventions to prevent HIV transmission and acquisition by reducing HIV-related risk behaviors and increasing protective behaviors. Additional funding requested in this By-Pass budget would permit NIH to sponsor studies of prevention strategies that could be implemented in racial and ethnic communities with high incidence of HIV infection and in groups disproportionately affected, such as young women of color and men who have sex with men. Funds above the FY 2008 Enacted level are also needed to allow adequate support for implementation or operational research that fosters the scale-up and use of existing efficacious HIV prevention interventions.
Therapeutics
NIH supports a comprehensive therapeutics research program to design, develop, and test drugs and drug regimens to prevent and treat HIV infection and its associated co-infections and co-morbidities. This By-Pass budget requests $730 million for HIV therapeutics research, an $87 million increase (13.5 percent) over the FY 2008 Enacted level.
NIH-supported research demonstrated the effectiveness of antiretroviral therapy to reduce mother-to-child HIV transmission. As a result of the implementation of these regimens, less than 200 HIV-infected babies are born each year in the United States. NIH is continuing to develop regimens that can be implemented in resource-constrained nations, including strategies to prevent transmission associated with breast-feeding. Antiretroviral treatment has resulted in improved immune function in patients who are able to adhere to the treatment regimens and tolerate the toxicities associated with antiretroviral drugs; and it has delayed the progression of HIV disease, extending the time between initial infection and the development of AIDS. However, epidemiologic studies have demonstrated that HIV-infected individuals are experiencing co-infections, including tuberculosis and hepatitis C, and co-morbidities associated with long-term HIV disease, such as malignancies, metabolic disorders, cardiovascular disease, and neurologic disorders. These complications result in more deaths occurring from liver failure, kidney disease, cardiovascular complications, and malignancies in this patient population compared to uninfected individuals.
Budget Policy
This By-Pass budget requests increased funds for research aimed at establishing a better understanding of the underlying biology of these HIV-associated conditions. This research is critical to development of better prevention and treatment strategies. At the FY 2008 Enacted level, NIH will be unable to provide necessary funding for basic and clinical studies on the increasing incidence of AIDS-related cardiovascular disease, diabetes, and malignancies, additional studies on the pathogenesis of HIV and Hepatitis C co-infection, and critical studies on metabolic abnormalities associated with HIV disease and long-term antiretroviral treatment.
Although improved therapeutic regimens for the treatment of AIDS and AIDS-associated co-infections and co-morbidities are urgently needed, particularly regimens that can be deployed in resource-limited settings, funding levels in this area have been significantly decreased over the past several years in this area in order to provide increased funding for HIV prevention research. This By-Pass budget requests additional funds to begin to restore those funds and expand support for the development of better lead compounds, drugs and therapeutic regimens that are less toxic and have fewer side effects, limit the development of drug resistance, enter viral reservoirs to inhibit viral replication, promote easier adherence, and are more readily accessible. Additional funds are critical for the development of therapeutic regimens that can be implemented in international settings to address the global impact and continued spread of the AIDS pandemic in both developed and developing nations. Thus, this By-Pass budget requests additional funds to support the infrastructure necessary for the conduct of perinatal, pediatric, and maternal clinical studies that will place a greater emphasis on sites in developing countries.
Etiology and Pathogenesis
NIH supports a comprehensive portfolio of research focused on gaining a better understanding of how HIV infection is established and maintained and what causes the associated profound immune deficiency and severe clinical complications. This By-Pass budget requests $807 million for this essential area of research, representing a $105 million increase (15 percent) over the FY 2008 Enacted level.
The results from recent microbicide and vaccine clinical studies have revealed gaps in the knowledge and understanding of HIV etiology and pathogenesis, particularly with regards to host immune responses and how HIV interacts with and transverses mucosal surfaces.
Budget Policy
Without the funds requested in this By-Pass budget, NIH will be unable to provide additional support for essential investigator-initiated basic research, including initiatives addressing the important pathogenic mechanisms more commonly observed in women, children, and adolescents infected with HIV. The results of such studies are critical for our efforts to prevent and control HIV infection and disease progression.
This By-Pass budget requests additional funds above the FY 2008 Enacted level to support an expansion of research to pursue novel ideas to better understand the normal development and functioning of the human immune system. These studies are crucial to answering essential questions about HIV pathogenesis and disease progression and the development of new and better treatments and prevention strategies.
Without these additional funds, research will be inadequate to address these questions, including the role of specific HIV proteins in the viral life cycle; the primary modes of HIV transmission between cells and between individuals; how the immune system controls the infection and disease progression; the mechanisms involved in cell injury and death in the immune, nervous, and other organ systems; host factors and cofactors that influence primary infection and disease course; and the relationship of HIV infection to its associated malignancies, opportunistic infections and co-infections, neurological impairments, and metabolic disturbances.
NIH-supported genomics studies recently identified genetic factors influencing the rate of viral suppression and the pace of HIV disease progression. This By-Pass Budget requests funding to launch a major new multi-year intramural/extramural program devoted to research on HIV and the human genome. This new program would allow NIH to capitalize on these new research findings and other recent advances in genomic and proteomic technologies that could lead to improved HIV therapies and provide new targets for vaccine, microbicide, and therapeutics development. NIH will be unable to initiate this critical multi-year program without a significant increase in funds over the FY 2008 Enacted level.
Natural History and Epidemiology
Natural history and epidemiologic research is needed to monitor epidemic trends, develop and evaluate prevention modalities, follow the changing clinical manifestations of HIV disease in different populations, and measure the effects of treatment regimens. This By-Pass budget requests $259 million in this scientific area, a $24 million (10.2 percent) increase over the FY 2008 Enacted level.
Epidemiologic research is instrumental in identifying and describing AIDS-related co-morbidities, disentangling effects related to treatment from those related to HIV disease itself. NIH researchers in two recent clinical trials demonstrated that heterosexual HIV acquisition was reduced by 50 percent in adult males who had been medically circumcised. These findings are of significant public health importance for HIV prevention. Ultimately, increased adult male circumcision could lead to fewer infections in women in those areas of the world where HIV is spread primarily through heterosexual intercourse.
Budget Policy
Additional funds requested in this By-Pass budget above the FY 2008 Enacted level will support research in domestic and international settings to examine HIV transmission, HIV/AIDS disease progression (including the occurrence of co-infections and opportunistic infections, malignancies, metabolic complications, neurological and behavioral dysfunctions), the development of other HIV/AIDS-related conditions, and improved methodologies to support this research. This budget requests additional funds for translational research in international settings to define the optimal parameters of treatment and care to achieve the best outcomes. Funds also are requested to support an evaluation of the community effectiveness of HIV prevention interventions.
Funding has been significant decreased in this area over the past several years in order to provide increased funding for HIV prevention research. This By-Pass budget requests additional funds to begin to restore those funds and expand support for critical additional support for epidemiologic studies to investigate the mechanisms of disease progression, the impact of therapy in changing the spectrum of HIV disease, and the causes of death. Additional funding is also requested to address the urgent need to enroll new study participants, particularly those manifesting new complications of long-term infection and therapy, in the Multicenter AIDS Cohort Study. Sufficient funding is not possible for these important studies without an increase over the FY 2008 Enacted level. Increased funds also are requested in this budget to continue to conduct research on circumcision as well as the development of other new and novel prevention strategies.
Training, Infrastructure and Capacity-building
This By-Pass budget requests $237 million for training, infrastructure, and capacity building, an increase of $44 million (22.8 percent) over the FY 2008 Enacted level.
Budget Policy
These funds are requested to support efforts to increase the supply of non-human primates, particularly rhesus macaques, for AIDS research and other areas of biomedical research both in the United States and abroad. In particular, this By-Pass budget requests critical additional funds for construction and renovation of the primate research centers, which are national resources. Additional funds are also requested to continue the expansion of NIH-funded HIV research globally, which has necessitated the development of research infrastructure in many locations, including resource-limited settings in Africa, the Caribbean, India, and Asia. This budget requests additional funds to support training programs for U.S. and international researchers to build the critical capacity to conduct AIDS research both in minority communities in the United States and in developing countries.
Information Dissemination
Effective information dissemination approaches are integral to HIV prevention and treatment efforts and critical in light of the continuing advent of new and complex antiretroviral treatment regimens, issues related to adherence to prescribed treatments, and the need to translate behavioral and social prevention approaches into practice. This By-Pass budget requests $30 million for information dissemination efforts, an increase of $4 million (15.4 percent) over the FY 2008 Enacted level.
The changing pandemic and the increasing number of HIV infections in specific population groups, such as racial and ethnic populations and women, highlight the need to disseminate HIV research findings and other related information to communities at risk. The flow of information among researchers, health care providers, and the affected communities represents new opportunities to rapidly translate research results into practice and to shape future research directions.
Budget Policy
This By-Pass budget requests additional funding to support: initiatives to enhance dissemination of research findings; develop and distribute state-of-the art treatment guidelines; and enhance recruitment and retention of participants in clinical studies, including women and minorities.
This By-pass budget also requests additional funds for a program to develop specialized tools for HIV sequence analysis through the National Center for Biotechnology Information. At the FY 2008 Enacted level, NIH is unable to provide increased funds to enable additional researchers to access these important resources and more fully utilize data from genomic studies.
Supporting Documents
The OAR is providing the following materials in support of this request:
- FY 2009 Trans-NIH Plan for HIV-Related Research
- NIH AIDS Research Funding Table by Scientific Area of Emphasis
- NIH AIDS Research Mechanism Table
Office of AIDS Research FY 2009 By-Pass Funding by the Areas of Emphasis of the Trans-NIH Plan for HIV-Related Research (Dollars in millions)
| Area of Emphasis | FY 2005 Actual |
FY 2006 Actual |
FY 2007 Actual |
FY 2008 Estimate |
FY 2009 By-Pass |
Percent Change ’08 to ’09 |
|---|---|---|---|---|---|---|
| HIV Microbicides1 | — | $86 | $97 | $103 | $128 | 24.3 |
| Vaccines | $509 | 581 | 583 | 593 | 681 | 14.8 |
| Behavioral and Social Science | 418 | 406 | 420 | 418 | 478 | 14.4 |
| Therapeutics | 732 | 635 | 656 | 643 | 730 | 13.5 |
| Etiology and Pathogenesis | 742 | 716 | 693 | 702 | 807 | 15.0 |
| Natural History and Epidemiology | 297 | 270 | 239 | 235 | 259 | 10.2 |
| Training, Infrastructure, and Capacity Building | 169 | 161 | 192 | 193 | 237 | 22.8 |
| Information Dissemination | 43 | 28 | 26 | 26 | 30 | 15.4 |
| Subtotal | 2,910 | 2,883 | 2,906 | 2,913 | 3,350 | 15.0 |
| Roadmap | 11 | 19 | — | — | — | — |
| Total | $2,921 | $2,902 | $2,906 | $2,913 | $3,350 | 15.0 |
1 Beginning in FY 2008, HIV Microbicides will be a separate activity. Dollars for HIV Microbicides were previously included within other science areas, such as Therapeutics, Etiology and Pathogenesis, Behavioral and Social Science and Vaccines. The FY 2006 and FY 2007 amounts are comparable budget figures.
National Institutes of Health Office of AIDS Research FY 2009 By-Pass Summary Mechanism (Dollars in thousands)
| FY 2007 Actual | FY 2008 Enacted | FY 2009 By-Pass | ||||
|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | |
| Research Projects | ||||||
| Noncompeting | 1,950 | $1,157,109 | 1,912 | $1,207,757 | 2,049 | $1,293,649 |
| Administrative supplements | (141) | 53,744 | (104) | 43,565 | (131) | 68,963 |
| Competing | 647 | 350,803 | 762 | 321,086 | 975 | 451,620 |
| Subtotal, RPGs | 2,597 | 1,561,656 | 2,674 | 1,572,408 | 3,024 | 1,814,232 |
| SBIR/STTR | 69 | 31,220 | 66 | 31,201 | 67 | 38,992 |
| Total, RPGs | 2,666 | 1,592,876 | 2,740 | 1,603,609 | 3,091 | 1,853,224 |
| Research Centers | ||||||
| Specialized/comprehensive | 54 | 122,458 | 55 | 124,140 | 56 | 139,875 |
| Clinical research | 5 | 44,215 | 9 | 52,366 | 8 | 61,604 |
| Biotechnology | 1 | 3,450 | 3 | 3,958 | 3 | 4,694 |
| Comparative medicine | 16 | 57,982 | 15 | 60,144 | 17 | 72,382 |
| Research centers in minority institutions | 3 | 10,213 | 3 | 11,830 | 3 | 12,871 |
| Subtotal, Centers | 79 | 238,318 | 85 | 252,438 | 87 | 291,426 |
| Other Research | ||||||
| Research careers | 276 | 38,784 | 275 | 38,870 | 290 | 49,661 |
| Cancer education | 15 | 15 | 38 | |||
| Cooperative clinical research | 13 | 28,016 | 13 | 25,650 | 14 | 34,600 |
| Biomedical research support | 1 | 1,961 | 1,462 | 1 | 1,535 | |
| Minority biomedical research support | 1 | 311 | 1 | 311 | 2 | 359 |
| Other | 134 | 62,371 | 122 | 63,483 | 133 | 75,121 |
| Subtotal, Other Research | 425 | 131,458 | 411 | 129,791 | 440 | 161,314 |
| Total, Research Grants | 3,170 | 1,962,652 | 3,236 | 1,985,838 | 3,618 | 2,305,964 |
| Training | FTTPs | FTTPs | FTTPs | |||
| Individual | 83 | 3,290 | 82 | 3,294 | 86 | 3,808 |
| Institutional | 738 | 32,519 | 725 | 32,884 | 761 | 42,854 |
| Total, Training | 821 | 35,809 | 807 | 36,178 | 847 | 46,662 |
| Research & development contracts | 226 | 457,634 | 222 | 443,089 | 180 | 501,644 |
| (SBIR/STTR) | (4) | (190) | (4) | (186) | (4) | (214) |
| Intramural research | 292,545 | 288,034 | 322,900 | |||
| Research management and support | 96,789 | 98,449 | 108,293 | |||
| Construction | — | — | — | |||
| Library of Medicine | ||||||
| Office of the Director | 60,359 | 61,757 | 64,884 | |||
| Buildings and Facilities | ||||||
| Total, Budget Authority | 2,905,788 | 2,913,345 | 3,350,347 | |||