 |

Studies Make Case for Finasteride to Prevent Prostate Cancer
The initial results from the largest completed prostate cancer prevention trial appear to have underestimated the benefits and overestimated the potential risks of finasteride, according to three new analyses of data from the trial. These results bolster the case for finasteride as a preventive agent against prostate cancer, say the studies' leaders.
Results from two of the analyses were presented on May 18 at the American Urology Association annual meeting in Orlando, FL, and all three appeared online May 18 in Cancer Prevention Research.
Read more 


Potent Social Forces Influence Smoking Behavior
Friends and family have a powerful influence on whether a person quits smoking, and the decision to stop smoking can "spread" from one person to another in a social network, new research suggests. The findings are from a detailed analysis of smoking behavior in more than 12,000 individuals who were followed for 32 years, from 1971 to 2003, as part of the Framingham Heart Study.
In 1971, the places smokers and nonsmokers held in the social network were indistinguishable. But three decades later, societal views of smoking have changed, and smokers are increasingly at the periphery of social networks and aligned largely with other smokers, according to results published in the May 22 New England Journal of Medicine. Read more 
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

Studies Make Case for Finasteride to Prevent Prostate Cancer
The initial results from the largest completed prostate cancer prevention trial appear to have underestimated the benefits and overestimated the potential risks of finasteride, according to three new analyses of data from the trial. These results bolster the case for finasteride as a preventive agent against prostate cancer, say the studies' leaders.
Results from two of the analyses were presented on May 18 at the American Urology Association annual meeting in Orlando, FL, and all three appeared online May 18 in Cancer Prevention Research.
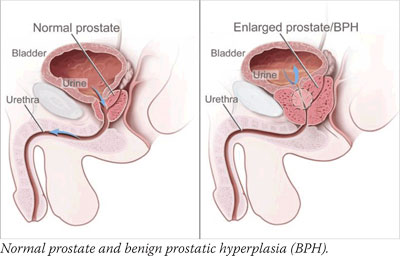
Initial results from that trial, the nearly 19,000-participant Prostate Cancer Prevention Trial (PCPT), were published in 2003 and showed that men who took 5 mg of finasteride daily for 7 years had a 25 percent reduced risk of developing prostate cancer compared with men taking a placebo.
However, finasteride treatment was also associated with a small but statistically significant increased risk for developing high-grade prostate cancers, those with Gleason scores of 7 to 10. And because the preventive benefit was the result of a reduction of non-high grade cancers, those with a Gleason score of 6 or less, some prostate cancer researchers argued that finasteride only prevents indolent cancers that would never require treatment, explains Dr. Ian Thompson, chair of the Department of Urology at the University of Texas Health Science Center at San Antonio and a senior author on two of the new analyses.
Neither conclusion, he says, now appears to be accurate.
"We've now shown that the cancers prevented by finasteride are often clinically significant, the same kind of cancers that lead to surgery," Dr. Thompson says. "In addition, we showed a 28 percent reduction of high-grade cancer with finasteride."
In a related editorial in Cancer Prevention Research, Drs. Christopher Logothetis and Paul Schellhammer, from University of Texas M.D. Anderson Cancer Center and Eastern Virginia Medical School, respectively, lauded the analyses, arguing that the results demonstrate that "the promise of prostate cancer prevention is a reality."
Two of the analyses were conducted independently, using 500 prostatectomy samples from the more than 2,000 patients diagnosed with cancer in the PCPT, to estimate the "true rate" of high-grade disease in the two study arms (finasteride and placebo). The analyses, one led by Dr. Mary Redman from Fred Hutchinson Cancer Research Center and the other by NCI's Dr. Paul Pinsky, used statistical modeling to extrapolate the Gleason scores at prostatectomy to the larger study population. Prostatectomy is the gold-standard for determining Gleason scores. Both analyses adjusted for the fact that, as recent studies have shown, finasteride increases the sensitivity of both prostate-specific antigen (PSA) testing and needle biopsy for detecting high-grade cancer.
No overall increase in high-grade disease associated with finasteride was found by either analysis, Dr. Thompson stresses. Instead, they found that finasteride had a modest protective effect, driven by the reduction in tumors scored as Gleason 7. Because of the limited number of cancers scored as Gleason 8 to 10, says Dr. Howard Parnes from NCI's Division of Cancer Prevention, it's not possible to draw firm conclusions about finasteride's affect on such tumors.
The third analysis, conducted by Dr. Scott Lucia from the University of Colorado Denver and colleagues, addressed whether finasteride prevents "clinically significant" prostate cancer by examining the extent of cancer present in the PCPT biopsy specimens of Gleason score 6 cancers - again, the cancers that finasteride was shown to inhibit in the PCPT. They assessed biopsy samples according to two different sets of criteria that can be used to guide treatment decisions. Sixty percent of tumors given a Gleason score of 6 or less were clinically significant by these criteria.
More than 90 percent of men whose prostate biopsies have Gleason scores of 6 opt to receive immediate treatment, explains Dr. Parnes, a co-author on two of the analyses. So, regardless of whether they would be considered clinically significant by these biopsy criteria, he says, "We shouldn't overlook the importance of preventing the so-called 'clinically insignificant' tumors."
Finasteride is not yet approved by the Food and Drug Administration (FDA) for prostate cancer prevention, Dr. Parnes cautions. "However, men committed to regular screening and those with benign prostatic hyperplasia (BPH) may want to discuss this option with their physicians," he adds. Finasteride is FDA-approved for treating BPH symptoms and, at a lower dose, for reducing hair loss.
FDA approval aside, there are other factors holding finasteride back, says Dr. Brantley Thrasher, chair of Urology at the University of Kansas Medical Center.
Urologists have become "acclimated" to the idea that finasteride increases the risk of high-grade disease, he says. And it's more than that. "Nobody has worked out which patients we should be recommending finasteride to," Dr. Thrasher adds. "I think it will be an uphill battle to get wider adoption."
The AUA and American Society of Clinical Oncology are in the process of developing guidance on finasteride use for prostate cancer prevention, Dr. Parnes says.
—Carmen Phillips
|
 |
 |
Potent Social Forces Influence Smoking Behavior
 |
 |

ASCO Annual Meeting News
Don't miss the next issue of the NCI Cancer Bulletin on June 10, which will feature the latest research news from the 2008 annual meeting of the American Society of Clinical Oncology.

|
 |
|
|
Friends and family have a powerful influence on whether a person quits smoking, and the decision to stop smoking can "spread" from one person to another in a social network, new research suggests. The findings are from a detailed analysis of smoking behavior in more than 12,000 individuals who were followed for 32 years, from 1971 to 2003, as part of the Framingham Heart Study.
In 1971, the places smokers and nonsmokers held in the social network were indistinguishable. But three decades later, societal views of smoking have changed, and smokers are increasingly at the periphery of social networks and aligned largely with other smokers, according to results published in the May 22 New England Journal of Medicine.
The authors, Drs. Nicholas Christakis of Harvard Medical School and James Fowler of the University of California, San Diego, reported last year that social networks may strongly influence obesity. In the current study, the authors demonstrate that decisions to quit smoking often reflect changes made by groups of people connected to each other, both directly and indirectly. For example, when a spouse quit smoking, the partner's chances of smoking decreased by 67 percent; friends who quit smoking decreased one another's chance of smoking by 36 percent.
 |
 |
NCI Cancer Bulletin Introduces New Feature |
 |
| Beginning with this issue, a new feature called "Profiles in Cancer Research"; will appear periodically in the NCI Cancer Bulletin. The feature will profile individual scientists, investigators, and clinicians who work at NCI or are funded by NCI. To read this issue's inaugural profile of Dr. Electra Paskett, a cancer epidemiologist at Ohio State University, please go to Profiles in Cancer Research. |
|
"People are connected, and so their health is connected," the researchers write, adding that "cessation of smoking in one person appears to be highly relevant to the smoking behavior of others nearby in the social network." They also suggest that the person-to-person spread of smoking cessation has been a factor in the significant decline in smoking seen in recent decades.
The network phenomenon could potentially be exploited to spread positive health behavior, the study concludes. Along these lines, collective interventions may be more effective than individual interventions, and public health strategies to encourage smoking cessation may be more cost-effective than initially thought, since health improvements in one person may well spread to others.
MRI May Contribute to Rising Mastectomy Rates
The number of mastectomies performed at the Mayo Clinic in Minnesota for women with early stage breast cancer jumped by 13 percent between 2003 and 2006, rising from 30 percent to 43 percent. A new study suggests that the introduction of preoperative breast magnetic resonance imaging (MRI), which is more sensitive than traditional mammography, may have been a factor.
An analysis of more than 5,400 women with early stage breast cancer who had surgery at the Mayo Clinic between 1997 and 2006 showed that women who received preoperative MRI were significantly more likely to undergo mastectomy than those who did not, though mastectomy rates rose in both groups. Co-author Dr. Matthew P. Goetz recently briefed the media on the findings, which will be presented next month at the American Society of Clinical Oncology annual meeting.
Preoperative breast MRI can detect cancer in more than one part of the breast, and this may lead physicians and patients to choose mastectomy over lumpectomy. About half of the lesions detected by MRI are not cancerous and only need to be monitored, but some women with these lesions may still choose mastectomy for various reasons, according to the researchers.
Mastectomy rates declined from 45 percent in 1997 to 30 percent in 2003, but then rose to 43 percent in 2006, the study found. During this period, the percentage of women who had breast MRI doubled to 22 percent. Half of the patients receiving MRI underwent mastectomy, compared with 38 percent of women who did not have MRI. Mastectomy rates also rose in women who did not have preoperative MRI (from 28 percent in 2003 to 41 percent in 2006).
Dr. Julie Gralow of the University of Washington, who moderated the briefing, cautioned that more research is needed to determine whether the additional surgeries improve outcomes and increase overall survival. A recent study showing an increase in preventive double mastectomies has similarly raised questions about the risks and benefits of these surgeries.
BRCA2 Linked to Prostate Cancer Incidence and Aggression
A study by researchers in Australia and Canada shows that BRCA2 mutation-positive men from BRCA mutation-positive families are at 3.5 times the risk of developing prostate cancer, and at higher risk of aggressive prostate cancer, compared with the general population. No increase in prostate cancer risk was observed in BRCA1 mutation-positive men in this study. The report appeared in the May 15 issue of Clinical Cancer Research.
The researchers examined 137 families that were known to have germline BRCA1 or BRCA2 mutations and that also had at least one male member who was diagnosed with prostate cancer. Among these families, 158 such men were candidates for the study. Lack of available prostate biopsies limited the analysis to 18 prostate cancer patients, 4 of whom carried germline BRCA1 mutations and 14 with germline BRCA2 mutations.
Loss of heterozygosity - in which one copy of a defective gene is inherited and the other copy becomes defective some time after birth, perhaps due to environmental factors - was detected in the prostate tumor tissue from 10 of the 14 BRCA2 carriers. The men whose cancers displayed loss of heterozygosity had exceptionally high Gleason scores of 7 to 9 (the median score was 9), and they had stage II or higher cancers.
"Although the sample size is small," the authors wrote, "these results indicate that BRCA2 has the hallmarks of a tumor suppressor and is the likely cause of the prostate cancer in a substantial portion of carriers who are diagnosed with the disease." The data add to prior information suggesting that prostate cancer may be part of the hereditary breast/ovarian cancer syndrome. The researchers added that there are no data to support a mechanism by which BRCA2 may cause prostate cancer.
The authors noted that BRCA2 mutation-positive (but not BRCA1 mutation-positive) men from hereditary breast/ovarian cancer families should be considered at significantly increased risk of prostate cancer by clinicians responsible for their care. They also suggest that additional evidence is required to formulate the specific screening advice and optimal management recommendations required to reduce the burden of prostate cancer in genetically at-risk men.
Vitamin D Not Associated with Decreased Prostate Cancer Risk
In a nested case-control study of participants from the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial, published online May 27 in the Journal of the National Cancer Institute, higher concentrations of serum 25-hydroxy vitamin D [25(OH)D], the primary form of vitamin D circulating in the bloodstream, were not associated with a decreased risk of prostate cancer. In fact, concentrations greater than the lowest fifth of the spectrum tended to be associated with an increased risk of aggressive disease.
Researchers in NCI's Division of Cancer Epidemiology and Genetics collected data on serum 25(OH)D concentrations from 749 white men diagnosed with prostate cancer and 781 controls, from samples taken at the beginning of their participation in the PLCO study (at baseline). All participants were taken from the PLCO trial screening arm, which includes annual standardized prostate cancer screening.
Serum 25(OH)D concentration was not associated with risk for prostate cancer overall, nor with non-aggressive disease; however, the researchers did see some evidence of an increased risk of aggressive disease associated with higher concentrations of vitamin D. They note that in their analyses not all the trends were statistically significant nor did the associations generally show a linear dose-dependence.
"In summary, results from this large prospective study of men who underwent standardized prostate cancer screening in the context of a screening trial do not support the hypothesis that higher serum vitamin D status is associated with decreased risk of prostate cancer," state the authors in an accompanying press release. "It raises the possibility that higher vitamin D level may be associated with increased risks for aggressive disease, [but] a clear monotonic dose-response relationship was lacking."
|
 |
 |

Guest Update by Dr. Daniela S. Gerhard
TCGA Moving Molecular Oncology Forward
 The Cancer Genome Atlas (TCGA), a collaborative project between NCI and the National Human Genome Research Institute (NHGRI), is at the mid-point of its 3-year pilot phase. The TCGA pilot is a truly integrative, multidisciplinary effort to develop and assess a framework for systematically identifying and characterizing the genomic changes associated with three cancer types: brain cancer (glioblastoma multiforme, or GBM), lung cancer (squamous cell carcinoma of the lung), and ovarian cancer (serous cystadenocarcinoma of the ovary).
The Cancer Genome Atlas (TCGA), a collaborative project between NCI and the National Human Genome Research Institute (NHGRI), is at the mid-point of its 3-year pilot phase. The TCGA pilot is a truly integrative, multidisciplinary effort to develop and assess a framework for systematically identifying and characterizing the genomic changes associated with three cancer types: brain cancer (glioblastoma multiforme, or GBM), lung cancer (squamous cell carcinoma of the lung), and ovarian cancer (serous cystadenocarcinoma of the ovary).
Already we are beginning to see the value of this project. Not only are new data being developed and shared with researchers around the world, but new technologies and tools are being developed that are allowing researchers to delve further into the molecular machinery of cancer with greater precision and efficiency.
For example, multiple technology platforms are being used at TCGA for molecular characterization and sequencing to interrogate tumor samples and their corresponding normal samples, ensuring that the data for each case are incredibly rich and as complete as possible. As the centers gain experience with some of the newer platforms, they learn how best to use them, both in terms of producing quality data as well as cost efficiency.
The different centers at the core of TCGA - which focus on characterization, sequencing, data collection and informatics, and technology development - are working in close concert integrating different data types, such as gene-expression signatures, microRNA-expression signatures, and methylation patterns, looking for new and more powerful ways to stratify tumors by subtypes, each of which may have different prognoses and require different treatment approaches. These data are then used to identify specific genes to sequence. The project team has designed TCGA to provide biomolecules (RNA and DNA) from a central facility to all TCGA centers to ensure uniform quality of the material.
To date, efforts with GBM are the furthest along, with more than 234 tumor cases (out of a planned 500) having undergone comprehensive characterization and a subset sequencing of 1,300 genes. This work is providing valuable insights into the biology of GBM and identifying potential new treatment avenues.
One example is an expanded understanding of the key signaling pathways involved in GBM. The integration of characterization data from the different TCGA labs has shown that not only is p53 - an important tumor suppressor gene - mutated more often in GBM than previously believed, but also that other key genes in the p53 pathway are often mutated, such that as many as 80 percent of GBM cases appear to have potentially important mutations in this pathway.
These findings suggest that other genes "downstream" of p53 could be valuable therapeutic targets. And this is just the first signaling pathway in GBM to be better elucidated.
It's important to stress that all TCGA centers have to immediately submit their raw data to the Data Coordinating Center, a database that is part of the cancer Biomedical Informatics Grid. The data are available to the scientific community, and all interested parties must sign agreements meant to strictly protect patient confidentiality.
The public spotlight is once again heavily focused on cancer, particularly brain cancer, following the malignant glioma diagnosis given to Senator Edward Kennedy, a long-time advocate for cancer research. Unfortunately, little progress has been made over the past several decades in the early detection and treatment of GBM. Our hope is that TCGA will help change that, allowing researchers to identify new methods for early detection, diagnosis, and treatment of GBM and the other cancers, and, in the process, recognize the true promise of molecular oncology.
Dr. Daniela S. Gerhard
Director, NCI Office of Cancer Genomics |
 |
 |
Following are newly released NCI research funding opportunities:
SBIR Phase II Bridge Awards to Accelerate the Development of New Cancer Therapies and Cancer Imaging Technologies toward Commercialization
Announcement Number: RFA-CA-08-021
Letter of Intent Receipt Dates: Aug. 19, 2008; Jan. 27, 2009.
Application Receipt Dates: Sept. 19, 2008; Feb. 27, 2009.
This funding opportunity will use the R44 award mechanism. For more information see http://researchportfolio.cancer.gov/initiativedetail.jsp?InitiativeID=3884. Inquiries: Dr. Andrew J. Kurtz - kurtza@mail.nih.gov.
|
 |
 |

A New Cancer Specialty: Follow-up for Long-term Survivors

 Diagnosed with inoperable Hodgkin lymphoma in 1978 at age 21, Eileen Gould received what was then state-of-the-art treatment: massive doses of radiation to the chest, abdomen, and pelvis. In addition, her spleen was removed. The treatment cured her lymphoma, which has never recurred. Diagnosed with inoperable Hodgkin lymphoma in 1978 at age 21, Eileen Gould received what was then state-of-the-art treatment: massive doses of radiation to the chest, abdomen, and pelvis. In addition, her spleen was removed. The treatment cured her lymphoma, which has never recurred.
Years later, she recounts, she was plagued by persistent fatigue and lack of endurance. Climbing a flight of stairs left her winded. Several doctors could find nothing wrong. Then she stumbled on an early online support group for long-term survivors of Hodgkin lymphoma, many of whom reported symptoms similar to hers.
"I thought, oh my gosh, I'm not crazy after all," says Eileen, 51, who lives in New York City. Nearly 20 years after her lymphoma diagnosis, a cardiologist confirmed that she had heart damage caused by large doses of radiation to her chest. Today Eileen lives with multiple chronic and late effects from the treatment that saved her life, including early menopause, hypothyroidism, chronic orthostatic hypotension, and a compromised immune system due to the removal of her spleen. She's had basal-cell skin cancers and in situ (noninvasive) breast cancer.
 |
|
Eileen is one of an estimated 1.5 million Americans alive today who were diagnosed with cancer 20 or more years ago. Roughly seven million people have survived a cancer diagnosis for at least 5 years. Data suggest that a variable proportion of these survivors already have or are likely to develop one or more chronic health problems that directly result from the cancer treatments they received. Now, to address their needs, a new specialty area in cancer treatment and research - follow-up care for long-term survivors - has begun to emerge at a number of cancer care facilities around the country.
Decades of research on survivors of childhood cancer have shown that the effects of cancer and its treatment can last a lifetime. The nature of these effects varies widely and depends on many factors, including the type of cancer treated, the way it was treated, and the patient's age when treated. More recently, studies have begun to document long-term and late effects of cancer treatments in cancer survivors diagnosed and treated as adults. A 2005 Institute of Medicine report, From Cancer Patient to Cancer Survivor: Lost in Transition, noted that few survivors currently receive comprehensive, coordinated long-term follow-up care. Such care may reduce the occurrence or severity of late effects through screening and appropriate preventive or early interventions.
Many children's hospitals and cancer centers with pediatric oncology programs have long had dedicated follow-up clinics for survivors of childhood and adolescent cancer, but these programs often follow survivors only until they reach their early 20s. To date at least eight new programs, supported by grants from the Lance Armstrong Foundation as Survivorship Centers of Excellence, have been created around the country that focus on the needs of older survivors and on long-term follow-up for survivors of cancer diagnosed in adulthood. Eileen Gould is a patient of one such program, located at Memorial Sloan-Kettering Cancer Center (MSKCC).
Although the structure of these programs varies, common features include leadership by a doctor knowledgeable about cancer late effects, state-of-the-art screening tailored to each patient's specific late-effect risks, referrals to appropriate specialists, wellness education, and research to better characterize not only the follow-up care needs of long-term cancer survivors but also the most effective approaches to providing that care.
"Post-treatment care is a new and evolving area of research," says Julia Rowland, director of NCI's Office of Cancer Survivorship. "We are only just developing the evidence base for what long-term follow-up care should look like. Examining the impact of such programs on the cost of care, and more importantly on survivors' ultimate health-related and quality-of-life outcomes, must be a critical part of this research."
A challenge for many long-term cancer survivors is lack of access to the records documenting the treatment they received. Eileen is fortunate to have all of her records, which her oncologist sent to her when he retired from practice. Using these records, Dr. Kevin Oeffinger, her physician and director of the MSKCC program for adult survivors of childhood and adolescent cancer, prepared a plan for her that documents the type of cancer she was treated for, the doses of radiation she received, the long-term or late effects that can result from that treatment, and the types and frequency of screening and monitoring she should receive. This approach is in line with what the IOM report described as "survivorship care plans," which it recommended for every cancer patient completing primary treatment.
"It's enormously helpful when I go to see my other doctors," says Eileen.
An avid tennis player before her cancer diagnosis, Eileen says she still enjoys an occasional doubles match. Despite her many health challenges, she says she's grateful to have survived this long. "I was so happy when I reached my 50th birthday - I never thought it would happen."
—Eleanor Mayfield
|
 |
 |

House Panel Holds Hearing on Breast Cancer and Environment Legislation
 |
|
Last week, U.S. Senator Edward M. Kennedy of Massachusetts, a longtime champion of cancer research, was diagnosed with brain cancer. In recognition of an increase in public and media attention, NCI is highlighting the topic on the Cancer.gov home page. Please click this tile for more information on the disease:

|
|
The U.S. House Energy and Commerce Subcommittee on Health held a May 21 hearing on legislation (H.R. 1157) to foster additional research at NIH on the possible environmental causes of breast cancer. Subcommittee Chairman Frank Pallone (D-NJ) noted that the legislation had been introduced in several previous Congressional sessions, and he and the sponsors of the bill hoped it would be passed without further delay.
Dr. Deborah Winn, chief of the Clinical and Genetic Epidemiology Research Branch in NCI's Division of Cancer Control and Population Sciences, testified that although the House bill is "well intended," it could have the "unintended consequence of narrowing the field of inquiry and promoting an unwise use of precious resources by mandating a specific way of conducting Federal research in breast cancer."
She stated that NIH and NCI already have sufficient authority to address this research area, noting that in FY 2008, "We expect $60.7 million will be specifically spent on researching the role of the environment in breast cancer development at NCI, and $35 million will be spent at the National Institute of Environmental Health Sciences, totaling almost $100 million in support of this important area across NIH."
Chairman Pallone noted that NIH had previously agreed to several revisions of the Senate version of the legislation (S.579). Dr. Winn reiterated that NIH did not oppose the Senate bill. S.579 was passed by the Senate Health, Education, Labor and Pensions Committee on February 27, 2008. Another witness, Fran Visco, president of the National Breast Cancer Coalition, said her group had negotiated with NIH on the revisions to the Senate bill. She and several House subcommittee members expressed hope that, with House concurrence to those revisions, the legislation may be passed during the current congressional session.
|
 |
 |

 |
 |

Profiles in Cancer Research
Read about this new feature.

|
 |
|
|
Dr. Electra Paskett
Marion N. Rowley Professor of Cancer Research, The Ohio State University
 The eastern edge of Ohio is rimmed by a mountain range called the Appalachian Plateau, a densely wooded territory marked by rolling hills, streams, and stunning waterfalls. Most of the people who visit do so for the camping, fishing, hiking, or to tour historic landmarks that reflect the Native American and coal mining heritage.
The eastern edge of Ohio is rimmed by a mountain range called the Appalachian Plateau, a densely wooded territory marked by rolling hills, streams, and stunning waterfalls. Most of the people who visit do so for the camping, fishing, hiking, or to tour historic landmarks that reflect the Native American and coal mining heritage.
Dr. Electra Paskett and her colleagues at Ohio State University (OSU) come here for a different reason. In this part of Appalachia, where the job market is depressed and poverty runs deep, the use of widely available cancer screening tests is low and, in turn, the cancer incidence and mortality rates are distinctly higher than in other parts of the state. "There's a problem here," she says. "We need to understand why and then do something about it."
The residents who live here are a world away - both economically and culturally - from where Dr. Paskett was raised. She grew up in Manhattan, where her late father, who was Greek, worked as a concert violinist and conductor. Nonetheless, in the course of her life and research, Dr. Paskett has developed a profound commitment to the welfare of people in Appalachia and other underserved areas of the country where circumstantial factors contribute to cancer health disparities.
"This is really important for the work that we do," says Dr. Mira Katz, a colleague at OSU. "She can take off her 'Dr. Paskett' hat and has the ability to talk one on one with people from different communities to find out the best way to connect with them. She's there to really help people, and that comes through all the time, despite differences in race or gender or any other demographic factor."
The connection may exist because for Dr. Paskett, this work is personal. Her first breast tumor was diagnosed in 1997 on a routine mammogram. Despite the fact that her mother and grandmother both suffered from the disease, she says, "The diagnosis still came as a shock." After surgery and radiation, she developed lymphedema in her hand and arm. Four years after the initial diagnosis, she had an axillary node recurrence. The second round of surgery and radiation, this time with chemotherapy, caused her to develop an enlarged, weakened heart, commonly called cardiomyopathy.
Her training as a cancer researcher (at the University of Utah and University of Washington in Seattle) brought only some comfort during the experience. "I knew that the treatments were good," she says, "but I also knew what could happen." She learned quickly that her familiarity with the health care system helped her identify who could answer her questions, "But this made me feel more empathetic for everyone else who is not a faculty member and doesn't have as much knowledge as I do."
Despite the long-term effects of her treatment - now mitigated by compression garments to limit the lymphedema swelling and medications to offset her heart damage - her friends and colleagues, including young investigators who she mentors, say that cancer has not kept Dr. Paskett down. In fact, they all remark on her energy and enthusiasm, which have led her to expand her research into lymphedema prevention and treatment, as well as quality of life for breast cancer survivors.
"Not many researchers span the cancer control continuum as she does," says Dr. Julia Rowland, director of NCI's Office of Cancer Survivorship. "Most people focus only on one aspect, such as treatment or prevention, but she has considerable breadth. This is one of the things that makes her such a strong researcher. She is a classic investigator who asks good questions and is open to new ideas. Integrative health, the interplay between mind, body, and environment on health, diversity and cultural competence…She just gets it."
Those who know her also admire her ability to accomplish so much while also finding time to enjoy her husband and three sons. She says that the secret is working smart. "You have to have a balance between your family, your work, and yourself - hobbies and other things that you like to do." As an example, she makes time for exercise almost every morning, and takes her husband, a physician assistant, along on trips if work requires her to leave during his birthday or other special occasions. If she is invited to a speaking engagement in New York City, she usually accepts because it gives her the opportunity to visit her oldest son, who is a college sophomore there.
Dr. Paskett says that one of the things she enjoys so much about living and working in Ohio is the diversity of surrounding populations. She also enjoys the direction that her research program is heading. "We're bringing biology in with behavior to look at the effects of lifestyle interventions on disease markers," she says. She's especially enthusiastic about ongoing research within the OSU Center for Population Health and Health Disparities, which is one of eight centers that work together as a network to explore the complexity of health disparities.
"We're looking at the issue of stress and how it relates to cancer, as well as other diseases," she explains. A research group in Chicago had done previous work showing that stress in animals can be induced by isolation, and that this in turn increases their tumor burden. This relationship in humans is now being examined across the eight centers.
"On the south side of Chicago, we saw big parallels with the animal models," she says. "In economically depressed, very urban neighborhoods, African American women didn't want to go outside of their houses. And here in Appalachia, we saw the very same things. We spoke with mainly rural white women, most of who had an abnormal Pap and were positive for HPV, and they said, 'I can't leave my house. We have no transportation, we have no jobs, we have no money to do anything.' So it's very interesting to see that underserved populations from different backgrounds and regions share the same problems. That deep down, they're more alike than they are different."
—Brittany Moya Del Pino
|
 |
 |

Selenium to Prevent Recurrence of Colorectal Polyps
Name of the Trial
Phase III Randomized Study of Selenium in Patients with Adenomatous Colorectal Polyps (UARIZ-00-0430-01). See the protocol summary at http://www.cancer.gov/clinicaltrials/UARIZ-00-0430-01.
 Principal Investigator
Principal Investigator
Dr. M. Peter Lance, Arizona Cancer Center at University of Arizona Health Sciences Center
Why This Trial Is Important
The mineral selenium, found naturally in grains, meat, and other common foods, is being studied to see if it can help prevent several types of cancer. Proteins in the body that incorporate selenium have antioxidant properties and help repair damaged cells, which may reduce the risk of cancer. Although the relationship between selenium in the diet and cancer risk is unclear, some studies of selenium supplementation have yielded promising results. In particular, the Nutritional Prevention of Cancer Trial, designed to see if selenium supplements could prevent nonmelanoma skin cancer, found that the supplements were linked with reduced risks of lung, prostate, and colorectal cancer.
"That study was a major justification for doing a randomized controlled trial with a colorectal cancer-related endpoint," said Dr. Lance.
In this trial, patients who have a history of colorectal adenoma - noncancerous growths (polyps) found in the colon or rectum that can be precursors to colorectal cancer - will be randomly assigned to receive either daily selenium supplements or a placebo for 3 or 5 years. At the end of the supplementation period, patients will have a colonoscopy to check for adenoma recurrence.
Whether patients in the study are treated for 3 or 5 years is at the discretion of the treating physician; some patients at higher risk of adenoma recurrence need a surveillance colonoscopy at 3 years after adenoma removal, while lower risk patients will have a surveillance colonoscopy after 5 years.
The investigators plan to follow the patients for 5 years after the end of supplementation. In addition to seeing if patients taking selenium have a lower risk of adenoma recurrence and advanced adenomas (adenomas closer to becoming cancer), the trial will characterize any side effects observed with long-term, high-dose selenium supplementation.
For More Information
See the lists of entry criteria and trial contact information at http://www.cancer.gov/clinicaltrials/UARIZ-00-0430-01 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |
 In Memoriam: Longtime NCI Employee Judy Patt
In Memoriam: Longtime NCI Employee Judy Patt
Judy Patt, who worked in cancer communications at NCI for several decades, passed away May 21 at her son's home in New Jersey after a year-long battle with metastatic lung cancer.
"Judy was an invaluable resource to everyone who ever made contact with her at NCI and would always try to find a helpful way of solving whatever problem was at hand," said her former supervisor Mary Anne Bright, associate director of the Office of Public Information and Resource Management, upon learning the news.
Ms. Patt came to NIH in 1962 as a Bacteriologist/Microbiologist and stayed until 1968, moving on to join a research company where she worked for nearly 20 years. In 1987, she returned to NCI and accepted a career appointment as a technical writer in the Office of Cancer Communications.
In 1999 she became a public affairs specialist in the Office of Cancer Information, Communications, and Education, and her last position, starting in 2002, was as a public health advisor for the Office of Communications Media and Public Communications in the Cancer Information Service Branch.
Ms. Patt received numerous awards for performance and special acts of service throughout her years of service, and she will be greatly missed by all of her co-workers, especially those in the Cancer Information Service Branch with whom she interacted on a daily basis.
Spring Research Festival Held at NCI-Frederick
The National Cancer Institute at Frederick (NCI-Frederick) held its 12th Annual Spring Research Festival May 14-15, 2008. The event, co-sponsored by the U.S. Army Medical Research and Materiel Command at Fort Detrick, featured scientific posters, a health and safety exposition, education information, and commercial exhibits featuring the latest scientific equipment and technologies.
In his keynote address, NCI Director Dr. John E. Niederhuber emphasized the uniqueness of the Frederick campus. NCI-Frederick is both a government-owned, contractor-operated facility and the nation's only Federally Funded Research and Development Center dedicated solely to biomedical research.
The annual research festival is open to the NIH and the Fort Detrick communities as well as the general public.
NCI Cancer Bulletin Writer Wins NAGC Award
The National Association of Government Communicators awarded NCI Cancer Bulletin senior writer Carmen Phillips a 2008 Blue Pencil Award. He received first place in the Writer's Portfolio category for the following collection of articles:
A New Tobacco Threat?
Do Rare Cancer Cells Have a Tale to Tell?
The Mathematics of Cancer
Mr. Phillips' work was selected from several hundred submissions in that category.
 |
 |

 NCI@ASCO
NCI@ASCO
Join NCI Director Dr. John E. Niederhuber at the American Society of Clinical Oncology (ASCO) annual meeting on Saturday, May 31 at 10:00 a.m., when he presents remarks during the opening session.
Meet the Experts
Learn about NCI's programs and Web sites at the NCI exhibit during the ASCO annual meeting, May 31-June 2.
Saturday, May 31
10:00 a.m.-11:00 a.m.,
1:00 p.m.-2:00 p.m.
Treatment of Tumors with TNF-alpha Inhibitors: A collaboration between NCI and NIAID
Sunday, June 1
10:00 a.m.-11:00 a.m.,
1:00 p.m.-2:00 p.m.
Treatment of Tumors with TNF-alpha Inhibitors: A collaboration between NCI and NIAID
11:00 a.m.-12:00 p.m.
Specialized Programs of Research Excellence (SPOREs)
Monday, June 2
10:00 a.m.-11:00 a.m.
Bringing Biomarkers to Clinical Practice
11:00 a.m.-12:00 p.m.,
1:00 p.m.-2:00 p.m.
Treatment of Tumors with TNF-alpha Inhibitors: A collaboration between NCI and NIAID

|
 |
|
|
| |
 |
 |

NCI Partners with Canary Foundation on Prostate Cancer Study
 NCI and the Canary Foundation, a nonprofit organization that funds research on early cancer detection, have announced a partnership for the purpose of identifying and validating biomarkers for high-risk prostate cancer.
NCI and the Canary Foundation, a nonprofit organization that funds research on early cancer detection, have announced a partnership for the purpose of identifying and validating biomarkers for high-risk prostate cancer.
Diagnosis and treatment of prostate cancer has its challenges, including overtreatment for non-lethal cases and the many cases of lethal prostate cancer that are still missed by current screening strategies. For example, the prostate-specific antigen (PSA) test commonly used to screen for prostate cancer is controversial, particularly its use in older men. One reason is that the forms of prostate cancer that are detected by PSA testing late in life often progress slowly, as opposed to the more aggressive and often fatal forms of the disease that may occur earlier.
NCI and the Canary Foundation signed a memorandum of understanding with a joint goal of reducing overtreatment by identifying aggressive versus passive prostate tumors. The Foundation - working with NCI's Early Detection Research Network (EDRN) - has pledged $3 million to initiate a Prostate Active Surveillance Study (PASS) at six research institutions across the country. EDRN will establish a disease-specific version of the Common Data Elements, a biospecimen management system, and a protocol oversight program to expedite the storage and processing of patient information and biological specimens.
Additionally, the Canary Foundation and EDRN will jointly establish a Biomarker Evaluation Group to determine those biomarkers that are most promising for evaluation, using the biologic materials collected by the Canary Prostate Consortium.
"Both Canary and EDRN believe that early detection technologies will lead the way on finding better cancer treatments for patients," said Don Listwin, founder and CEO of the Canary Foundation. "In this specific study, we hope to identify biomarkers that will tell us which prostate cancers need to be aggressively treated, and which do not. Being able to collaborate with EDRN will help us achieve this goal much faster."
Dr. Sudhir Srivastava, chief of NCI's Cancer Biomarkers Research Group, noted: "We are delighted to join forces with the Canary Foundation. With prostate cancer being one of the major focus areas for Canary's cancer programs, and with EDRN's multiple ongoing studies related to the early detection of prostate cancer, we see this as a complementary and significant partnership."
The six institutions that will participate in the active surveillance study are Stanford University; University of California, San Francisco; University of British Columbia; University of Washington; Fred Hutchinson Cancer Research Center in Seattle; and University of Texas Health Science Center at San Antonio.
The Canary prostate team is headed by Dr. Peter Nelson, member of the Human Biology and Clinical Research Divisions at Fred Hutchinson and professor of oncology at the University of Washington. He is also a practicing medical oncologist, with a clinical specialty focused on the treatment of prostate cancer.
"We are proud to launch this new study with EDRN and with the participation of leading research institutes," said Dr. Nelson. "Through collaboration we can make bigger strides in providing better, more individualized treatment for prostate cancer patients."
—Bill Robinson
|
 |
|