Frequently Asked Questions - Full List
<< Back to FAQs
Prepare to Apply
Registration in Grants.gov
- What do I need to do before I can submit an application through
Grants.gov?
Grants.gov and eRA Commons registrations are required prior to application submission. Grants.gov registration provides the ability to submit applications electronically. eRA Commons registration allows NIH to receive applications electronically from Grants.gov and validate them against agency-specific business rules. It also provides a way for NIH and registered users to communicate electronically after submission. Assignment, review outcome and summary statement information is available through the eRA Commons.
- Does a Principal Investigator have to register in Grants.gov and
eRA Commons?
A Principal Investigator does NOT need to register in Grants.gov but MUST be registered in the eRA Commons prior to electronic submission of a grant application. The Principal Investigator (PI) registers in Commons through the organization’s Authorized Organizational Representative (also known as the Signing Official).
- When should applicant organizations begin the registration process?
To avoid any potential processing backlogs due to last minute registrations, applicants are highly encouraged to start the registration process at least four weeks prior to the grant submission date. New businesses [i.e. those applying to the Internal Revenue Service (IRS) for an Employer Identification Number (EIN) to complete the CCR registration] should start the process at least two months (eight weeks) prior to the grant submission date. If an applicant has started the eRA Commons registration process at least two weeks in advance of the submission date, NIH will consider it a “good faith” effort to prepare for electronic submission and the applicant will not be penalized for any NIH-caused registration processing delay. The applicant will, however, need to follow the established procedures for late submissions documented in the NIH Guide to Grants and Contracts at http://grants.nih.gov/grants/guide/notice-files/NOT-OD-06-086.html and describe the reason for the late application in the PHS 398 Cover Letter component of the application package.
Please note that Institutions/organizations must be registered with Grants.gov, as well. NIH will not make any allowances for submission delays due to incomplete Grants.gov registration.
Also see FAQs on Submission Deadline.
- What is involved in the Grants.gov registration process?
Applicant Organizations need to complete a one-time only registration process for Grants.gov that includes obtaining a Data Universal Numbering System (DUNS) number, registering in Central Contractor Registry (CCR) and registering in Grants.gov. Detailed steps for Grants.gov registration for both domestic and foreign organizations can be found at http://era.nih.gov/ElectronicReceipt/preparing_grantsgov_reg.htm . In addition, registration information can be found at the Grants.gov Get Registered webpage: http://www.grants.gov/applicants/get_registered.jsp. Also see Flow Chart of Grants.gov Registration Process (PDF - 25 KB)
Please note that this is a one-time only registration for all Federal agencies using Grants.gov. So if your organization has already completed the Grants.gov registration process to submit electronically for another Federal agency, a separate Grant.gov registration is not necessary for NIH submissions.
- Part of the Grants.gov process is registering in the Central Contractor
Registry (CCR). What is the CCR and how will an applicant organization know
if they have already registered or have successfully registered in the CCR?
Grants.gov requires that applicant organizations obtain a DUNS number and register with the Central Contractor Registration (CCR). CCR is a government-wide registry for vendors doing business with the Federal government. Grants.gov uses CCR to establish roles and IDs for those electronically applying for grants. In the future, the government anticipates requiring all grant applicants to use CCR whether applying for grants electronically or otherwise. To register in CCR, one needs a DUNS number.
To register in CCR: - Go to the CCR home page at www.ccr.gov
- Click on 'Register in CCR'
- You must have a Data Universal Numbering System (DUNS) Number in order to begin the registration process.
- Complete and submit the online registration.
- How will an applicant organization know if they are already registered
or have successfully registered in Grants.gov?
The Authorized Organizational Representative (AOR) of the organization should know if the organization has already completed the one-time only Grants.gov registration process. For more information on Grants.gov registration, see the Grants.gov Get Registered webpage: http://www.grants.gov/applicants/get_registered.jsp
- My organization already has a DUNS number. Do we need to establish
a different one for Grants.gov submissions?
Your organization will need to determine if the already established DUNS number is being used for grant applications. Keep in mind that applications to the NIH have required a DUNS number since October 1, 2003. So most applicant organizations have already fulfilled this registration step.
- Grants.gov requires a DUNS and CCR Registration. I'm also
a reviewer for NIH so now I'm required to have an individual DUNS & CCR
registration as well. Will I use my individual DUNS on applications
and my individual CCR registration?
No. When submitting applications through Grants.gov, the DUNS number of the applicant organization and the CCR registration of the Authorized Organizational Official of the applicant organization must be used. The DUNS number used during CCR registration must also match the DUNS number in the organization's profile (Institutional Profile File) in eRA Commons.
- I seem to be receiving a lot of unnecessary email solicitations
and spam after I registered at the Central Contract Registration (CCR) and
obtained a Data Universal Numbering System (DUNS) number for my organization
as part of the Grants.gov registration process. How can I prevent this spam?
The Central Contract Registration (CCR) includes this information on its website (http://www.bpn.gov/ccr/scripts/index.html):
“As a result of obtaining a DUNS number, you might be included on D&B’s marketing list that is sold to other companies [Dun and Bradstreet (D&B) is the commercial company that provides the DUNS number].”
If you do not want your name or company name included on this marketing list, D&B has asked that you contact them anytime at 1-866-705-5711 to request removal from that list.
Registration in Commons
- What is involved in the NIH eRA Commons registration process?
Applicant organizations submitting grants to NIH must complete a one-time, two-step registration in the NIH eRA Commons. For detailed steps, click on Detailed steps for Commons registration (PDF - 36 KB).
- Commons Registration for the Organization: To find out if an organization
is already Commons-registered, check this query 'List
of Commons Registered Organizations.'. For organizations that need
to register, go to the 'Grantee
Organization Registration' link on the eRA
Commons Homepage.
- Commons Registration for the Principal Investigator (PI): The individual
designated as the PI on the application must also be registered in
the Commons. The PI must hold a PI account and be affiliated with the
applicant organization. This registration must be done by an organization
official or their delegate who is already registered in the Commons.
To register PIs in the Commons, refer to the eRA Commons User Guide
found at: http://era.nih.gov/commons/index.cfm.
- When a Signing Official, Administrative Officer or Account Administrator
creates an account for a PI, the PI is sent an email to that effect
telling him or her to go to the Commons to verify their profile information.
- Who needs to be registered in the eRA Commons--just the Grantee
Institution, the Principal Investigator (PI), or all Senior/Key Persons?
The applicant organization, Signing Official (SO) and the PI must be registered in the NIH eRA Commons.
- Do the PI and
SO require separate accounts in Commons (even if the PI and SO are the
same person)?
Yes, both the PI and SO need separate accounts in Commons. Only an SO has the ability to 'reject' an application in Commons to address warnings or if the assembled application does not reflect the submitted application package due to eRA Commons or NIH system issues. If an SO is given a PI role, it overrides the SO’s privileges such as the ability to reject the application, submit eSNAPs or Just-In-Time information and request No Cost Extensions. Therefore, if you are the SO for your organization as well as a PI of the grant, you will need two separate accounts with different user names — one with SO authority and one with PI authority. When an institution is registered, an SO account is created. Log on to the account with the SO authority role and create another account with PI authority.
- I have an Internet Assisted Review (IAR) account. Will this
satisfy the requirement for an eRA Commons account?
The Principal Investigator must have a PI role on the eRA Commons account in addition to the Internet Assisted Review (IAR) role. The PI should work with the Signing Official to verify that the PI has a PI role. For PIs who are selected for an Internet Assisted Review role, an IAR authority is automatically added to their account once a Scientific Review Administrator enables them for a meeting. All other reviewers who have never served as PIs only have IAR authority.
- Does a PI who moves to another institution have to register
again in Commons?
No. The second institution should affiliate the PI's Commons account with their institution. A PI's Commons account follows the PI throughout the PI's career. The steps to affiliate a PD/PI to the applicant organization/institution are: - For consortium or subawards, do the sub-awardees need to be registered with eRA Commons and Grants.gov?
Sub-awardees are not required to register. However, we do encourage them to be proactive and register to be ready to serve as primary awardees in the future.
a. PD/PI gives Commons user ID and email address to the administrator of the applicant institution. (The email address must be the one that is contained in the Personal Profile for the PI.)
b. Administrator logs into the Commons. (The administrator can be the Signing Official, Administrative Official, or the Accounts Administrator.)
c. Administrator selects "Administration" tab and then "Accounts" tab.
d. Administrator selects "Create Affiliation" tab.
e. Administrator enters the Commons User ID and Email address into the appropriate fields and clicks "Submit."
Hardware
- What are the minimum hardware requirements for submitting grants
electronically to NIH?
Grants.gov, not NIH, specifies the hardware requirements since all electronic grant applications are submitted to Grants.gov and then retrieved by NIH.On the Grants.gov website, its Apply for Grants webpage states:
In order to view the downloaded application package, you will need to install the PureEdge Viewer (Windows EXE File). There are basic system requirements for using the PureEdge Viewer. If you are a non-Windows user, please refer to this support page.
The Grants.gov system requirements for Windows users are:
Windows 98, ME, NT 4.0, 2000, XP
500 MHz processor
128 MB of RAM
40 MB disk space
Web browser: Internet Explorer 5.01 or higher, Netscape Communicator 4.5 - 4.8, Netscape 6.1, 6.2 or 7.
Note: Grants.gov does not currently support Windows Vista.
Software
- What software do I need to have loaded before I can begin using
Grants.gov?
Applicants will need to download the PureEdge viewer free of charge from Grants.gov; see PureEdge Viewer on the Grants.gov website. The viewer will allow applicants to download application packages and guides.For NIH applicants, an application to create PDF files will be needed. On the Grants.gov Download Software webpage users will find a variety of PDF Conversion Tools for the applicant to use.
- What options are available to Macintosh users who rely on the Grants.gov form-based solution?
NIH is anticipating the arrival of the new Grants.gov 2007 solution that is based on Adobe forms and is platform independent. In the meantime, please continue to use the following proven options for Macintosh users:
- NIH-hosted
Citrix® servers: allow non-PC users to prepare and submit applications
using the PureEdge forms viewer. This service has been used successfully
by many applicants.
- PC-emulation software: commercially available products allow Mac users
to run the PureEdge viewer.
- IBM Workplace Forms (PureEdge) Viewer for Macintosh: Grants.gov provides
access to an IBM PureEdge viewer that is compatible with Macs. There are
some limitations to the viewer, so we strongly suggest that you read the
available documentation carefully before deciding whether using the viewer
is a good option for your specific circumstances. For information, visit
Grants.gov's IBM
Workplace Forms (PureEdge) Viewer for Macintosh.
- Commercial Service Providers: offer a wide range of platform independent services - from low-cost, single transaction options through full scale, end-to-end grants management solutions. You should coordinate with your institutions' grants office to explore these options further.
- NIH-hosted
Citrix® servers: allow non-PC users to prepare and submit applications
using the PureEdge forms viewer. This service has been used successfully
by many applicants.
- I am a Macintosh user. What tips should I keep in mind when using the Citrix server?
The free Citrix server is available to non-Windows users to remotely launch a Windows session and submit completed grant applications. Keep these tips from the Grants.gov website and fellow applicants in mind when using Citrix:
- You must download your Grant Application Package from Grants.gov prior
to using Citrix and save it with an ".xfd" extension. The package can be downloaded to a non-Windows machine; it just cannot be viewed or worked on directly without Citrix.
- If you close the Internet Explorer session you'll be disconnected from
the server and you'll have to reconnect again. Data will be lost if you
have not saved it.
- If you fail to use Citrix for more than 20 minutes the system will time
out and you will have to reconnect. Data will be lost if you have not saved
it.
- You must save your data often in case of loss of connection, or 20 minutes
or more of idle time.
- Save each version of your application with a different name.
- Save your application with file names no longer than 8 characters; filenames
should be simple with NO special characters.
- A limited amount of users may access the Citrix Server at one time.
- Log off when you are not working on your application package in order
to maximize the use of this server.
- Please be aware that you should wait until you are close to completing
your application package before you attach any documents to keep the size
of your file as small possible.
- Some Citrix users have experienced problems sharing files on shared
servers. If you are experiencing problems, save the application on your
local computer.
Macintosh System Requirements:
OS X Version 10.1, 10.2, 10.3, or 10.4.
128 MB of RAM
10 MB Disk Space
PowerPC Processor
For information:
Grants.gov website:
- Download Software http://www.grants.gov/agencies/asoftware.jsp#2
- Grants.gov Citrix Package Start-up Guide http://www.grants.gov/techlib/grantscitrixpackage.pdf (PDF - 175 KB)
- You must download your Grant Application Package from Grants.gov prior
to using Citrix and save it with an ".xfd" extension. The package can be downloaded to a non-Windows machine; it just cannot be viewed or worked on directly without Citrix.
Policy
- Can an applicant file using an SF424 (R&R) for a mechanism
that has not yet officially transitioned (i.e., start using SF424 (R&R)
now and stop using the PHS 398 entirely)?
Applicants cannot submit applications through Grants.gov on the SF424 (R&R) for mechanisms that have not yet transitioned. NIH systems will not be ready to accept them.
Find and Download Opportunity
- With electronic submission, all applications must be submitted
in response to a Funding Opportunity Announcement. How does one submit applications,
especially R01s, which previously came in unsolicited?
NIH and other HHS Agencies have developed generic Parent Funding Opportunity Announcements for use by applicants who wish to submit what were formerly termed “unsolicited” or "investigator-initiated" applications. See the Parent Announcements page for a list of current Parent Announcements and further information.
- Will NIH continue to use the NIH Guide to Grants and Contracts?
Yes. Opportunities will be published in both the NIH Guide and the Find section of Grants.gov.
- How does the Grants.gov system populate the field for CFDA
numbers?
The Grants.gov system allows NIH to leave this field blank. NIH staff will complete this field in the eRA system after submission.
- Should I look for an opportunity by entering the CFDA number
on Grants.gov?
On Grants.gov, applicants should search by Funding Opportunity Announcement number rather than CFDA. Note that NIH has made it easier for applicants by adding a button titled 'Apply for Grant Electronically' (see button below) to the NIH Guide for Grants & Contracts announcements that allows applicants to access the Grants.gov application package directly from the Guide.
- When I download an application package from the FOA and save it
for the first time locally, I get this warning message:
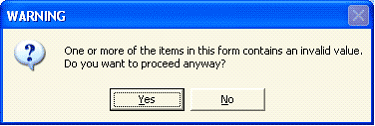
Is there something wrong with the forms?
No, the forms are OK. The message shows because no data has been entered yet. You will actually get this or a similar message every time you save until all data entry is complete. The applicant can ignore this error message until the final save before you submit. If you are ready to submit and are still getting this error message when you save, you should use the "Check Package for Errors" button to determine what may need to be fixed before submitting. If errors are found, a message box will appear telling you the total number of errors found and the details about the first one. Unfortunately there is no way to get a comprehensive list at this time. If you have five errors, you would have to fix the first one, and repeat the "check package for errors" process until all are corrected.
Prepare Application
Application Instructions
- Where is the SF424 (R&R) Application Guide available?
The application guide can be found on OER’s SF424 (R&R) Application and Electronic Submission Information page.
- Where will an applicant need to look to find application instructions?
As with our current business process, there are essentially two places an applicant looks for instructions. First is the application guide. This document will be posted with every Funding Opportunity Announcement (FOA) posted in Grants.gov. Second is the FOA itself. Program-specific application requirements will continue to be part of the actual announcement.
Resubmission, Revision, Renewal
The March 5 receipt date for renewal, resubmission, and revision R01 applications is right around the corner. Here are some FAQs to assist with issues specific to this deadline.
- NIH and Grants.gov seem to use different terminology for application type, how do I know which term is correct for my situation?
Grants.gov has brought us new terminology for the Type of Application field of the SF424 (R&R) Cover Component (box #8). NIH is trying to change all of its materials to correctly reflect the new terminology, but it will take some time. Please use the handy chart below as we work through this terminology change.
New Grants.gov Term Old NIH Term Notes New New An application that is submitted for funding for the first time. Includes multiple submission attempts within the same round. (Type 1) Renewal Competing Continuation Previous years of funding for the project have elapsed. Competing for additional years of funding to continue original project. (Type 2) Revision Competing Supplement Request for additional funds for a current award to expand the scope of work. Applicants should contact the awarding agency for advice on submitting any revision/supplement application. (Type 3) Resubmission Revision or Amended Application Application previously reviewed. A revised or amended application addresses reviewer feedback. (A1/A2) Continuation Progress Report NIH does not use the SF424 (R&R) for Continuation Applications. (Type 5; Progress Reports for Simplified Non-competing (SNAP) are submitted directly to eRA Commons for others paper is still submitted)
- What do I do if more than one application type seems to fit my situation?
In the PHS 398 paper world, applicants could identify more than one application type for a single application. However in the new SF424 (R&R) world, only one option can be selected. An easy rule of thumb is that any application that is submitted in response to review feedback should be marked as a resubmission. So, if an applicant is submitting a resubmission of a renewal or a resubmission of a revision, then resubmission should be chosen as the single application type.
- What do I put in the Federal Identifier field of the SF424 (R&R) cover component?
If "Type of Application" is "New", you can leave the Federal Identifier field blank on the first submission attempt. However, the Federal Identifier field becomes a required field when submitting a Changed/Corrected application to address errors/warnings. When submitting a Changed/Corrected "New" application, enter the Grants.gov tracking number of the previous submission attempt (e.g. GRANT00123456). If you are unable to find the tracking number, enter "N/A".
If "Type of Application" is "Renewal", "Revision" or "Resubmission", enter the IC and serial number of the prior application/award number (e.g. CA123456). For these types of applications, do not change the Federal Identifier field when submitting Changed/Corrected applications.
- When submitting an application again to address errors or warnings, how do I indicate on the form that the current submission supersedes the previous?
On the SF424 (R&R) cover component, box #1 Type of Submission should be set to "Application" on the initial application submission. Box #1 should be set to "Changed/Corrected" for all subsequent submissions of the same application to address errors or warnings.
Note that box #8 Type of Application remains the same from one submission attempt to the next within the same receipt deadline.
See the section of the application guide titled "Correcting Errors" for additional information.
- What part of the application/award number is the IC and serial number?
NIH's grant application/award numbers consist of the following parts:- A single-digit Application Type
- A three-digit Activity Code
- A two-letter IC Code
- A six-digit Serial Number
- A two-digit Grant Year (preceded by a dash to separate it from the serial number)
- Additional suffix information that may include the letter "S" and related number for a particular supplement record, the letter "A" and related number to identify an amendment and/or the letter "X" and related number to identify a fellowship's institutional allowance record.
For example, 3R01CA123456-04S1A1 would indicate an amendment (A1) to a supplemental (Type 3) application for a traditional research project (R01) referred to the National Cancer Institute (CA). The number further identifies the application serially as the 123456 new proposal submitted to the NCI, and indicates that this is the first supplemental application (S1) to the fourth year (-04) of the support to this project. In this example, the IC and serial number would be "CA123456".
Additional information on the NIH grant application/award identification numbering system can be found at: http://grants.nih.gov/grants/funding/ac.pdf (PDF - 622 KB).
- Is it OK to scan portions of the original PHS 398 application when submitting a revision, renewal or resubmission?
If you are making the move from paper to electronic forms, please resist the temptation to scan sections of the paper forms. There are times when scanning simply can't be avoided, but (when possible) it is best to work from the original documents that can be appropriately edited for the current submission, converted to PDF format and attached to the new application. Additional benefits of working from original documents include clearer images and the ability to extract text from the application image. (PDF Tips)
SF424 (R&R) Form
Application
Form
- Which form will applicants use to submit applications to NIH via
Grants.gov?
Applicants will use the Standard Form (SF) 424 family of forms. SF424 consolidates grant applications, related data and forms currently used by Federal grant-making agencies to enable applicants to use familiar forms regardless of the program or agency to which they are applying. The SF424 Research & Related (R&R) will become the government-wide data set for research grant applications. The SF424 (R&R) will replace the Public Health Service (PHS) 398 form at NIH.
- What do the new forms look like? Are samples available?
Samples of the SF424 (R&R) form are available from NIH’s SF424 (R&R) Application and Electronic Submission Information page at http://grants.nih.gov/grants/funding/424/index.htm. Remember, sample forms cannot be used for submission. Applicants must use the forms package downloaded from the actual funding opportunity announcement to successfully submit a grant application.
- What components make up the SF424 (R&R) grant package?
The SF424 (R&R) grant package includes the following components (included components will vary by mechanism): - R&R Application/Cover Component
- R&R Project/Performance Site Location(s) Component
- R&R Other Project Information Component
- R&R Senior/Key Person Component
- R&R Budget Component
- R&R Personal Data Component
- R&R Sub-award Budget Attachment Component
- Are SF424 components portable? Can components be reused for other
applications?
Currently there is no way to reuse the forms from one opportunity to another. Grants.gov hopes to have the functionality next year to import and export data for reuse with other applications.
- How will NIH collect information contained in the PHS398 form
that is not included in the SF424 (R&R) form set?
The SF424 accommodates agency-specific data collection. The following new application forms may be included with NIH's SF424 (R&R) grant packages: - PHS 398 Cover Letter File
- PHS 398 Cover Page Supplement
- PHS 398 Research Plan
- PHS 398 Modular Budget
- PHS 398 Checklist
- I see that some of the application components are labeled as "PHS
398". Will the reference to the "PHS398-specific" data elements
cause confusion to the applicants? Why not use "NIH"?
The use of the term PHS398 was chosen since that is the OMB-approved data collection instrument that gives NIH and other PHS agencies the authority to collect those specific items. Keep in mind that the data included in those components are only those items that are not included elsewhere in the SF424 (R&R). Using the more generic term PHS398 rather than "NIH" allows other HHS agencies that currently use the PHS398 to use these Grants.gov developed components as well.
- From time-to-time new application data requirements are necessary.
What will be the process to add such data elements to the SF424 (R&R)
application?
It will now be a two level decision process. If the data requirement effects the majority of agencies using the SF424 (R&R) application, then there will be a process by which new data elements are discussed and approved for addition to the SF424 (R&R). If the data requirement affects only the NIH, then we will request Office of Management and Budget permission to add it to the agency-specific data requirements.
- Has there been any input from the grantee community in developing
the SF424 (R&R)?
The development of the SF424 (R&R) began as a Federal-wide interagency project. Members of the Commons Working Group participated in testing of the SF 424 (R&R) in May 2005 and provided feedback to NIH. NIH-wide Focus groups have actively contributed to the discussion.
- Where is the budget justification located?
In the SF424 (R&R) detailed budget component, the budget justification is item K--a PDF upload. In the PHS398 Modular budget component, budget justifications for Personnel, Consortium and Additional Narrative are requested as separate PDF uploads as part of the Cumulative Budget Information.
- Where is Other Support located?
The SF424 (R&R) Senior/Key Person component includes an attachment upload for "Current and Pending Support", the equivalent of NIH's "Other Support". However applicants are being instructed not to use this upload. NIH will continue to collect Other Support information via the Just-In-Time process so it will not be requested as part of the initial application submission.
- The SF424 (R&R) Personal Data page includes very sensitive
personal data, like the Social Security Number. Is there concern about the
security of such data?
Grants.gov is a secure, reliable source to apply for Federal grants. However, since NIH requires that all PIs also be registered in the NIH eRA Commons before submitting through Grants.gov, NIH will already have all the pertinent personal information in their profile. Consequently, electronic submissions through Grants.gov will not include the Personal Data component.
- Once an application is submitted through Grants.gov, the data
is configured as an SF424 (R&R) application and stored in the Grant
Folder in an eRA database. Will this have any impact on the way the Center
for Scientific Review (CSR) refers applications to IC Referral Offices?
The referral guidelines for ICs and study sections will not be changed by this process. Of course the referral will be done using the electronic image, rather than paper. But at least at the beginning there will be no changes in the referral process. Division of Receipt and Referral staff will continue to make assignments for primary and dual ICs and review location (IC or CSR). Ultimately a knowledge management system may be involved in making suggestions for assignments but there still will be decisions made by scientifically trained staff.
- The SF424 (R&R) cover component requires you to enter your
Congressional District as well as the Congressional District of your project's
primary site. How do I locate my Congressional District?
One way to locate your Congressional District is to go to the U.S. House of Representatives website at http://www.house.gov/writerep/, select your state and enter your zip code. Then click the “Contact My Representative” button. The screen that pops up will list your representative as well as your congressional district.
Budget (including Subaward)
- Is a DUNS number required for every subaward/consortium organization?
Ideally yes. The 'Organization DUNS' is a required field on the 'Research & Related Budget' form, regardless of the budget "type"-project or subaward/consortium. However, at the subaward level, neither Grants.gov nor NIH currently validates on the accuracy of that field. For subaward organizations, eRA Commons only validates that the DUNS field contains a value and that the value is not the same DUNS number provided by the prime applicant. At this time the eRA Commons does not do any further validations on the accuracy of the number. So for now, if a subaward/consortium organization is unable to secure a DUNS number in time, then a value of nine zeros can be entered in the DUNS field on the subaward/consortium budget component. This requirement may change over time.
Remember that the prime applicant uses the R&R Subaward Budget Attachment form to generate a copy of the Research & Related Budget form that can be sent to the subaward/consortium organization, filled out, sent back to the prime and attached to the R&R Subaward Budget Attachment form.
- Is CCR & Grants.gov registration required for subaward/consortium institutions?
Subaward/consortium organizations need not register with CCR or Grants.gov, unless they plan to submit some day as an applicant organization. This requirement may change over time.
- Is a Commons registration required for every subaward/consortium organization?
Subaward/consortium organizations need not register in the eRA Commons, unless they plan to submit some day as an applicant organization. This requirement may change over time.
- For the Indirect Cost Rate (%) field in the budget form, I can
only enter up to 2 numbers. Our rate is 110%. How can I enter 110%?
Grants.gov is working on a solution. Until one is implemented, the recommended workaround is to split the Indirect Cost Rate into 2 lines on the budget form.
- Where should I enter the subawardee's indirect costs in the main project budget?
When a grant involves a subcontract, the total costs (Direct + Facilities & Administrative Costs) of all subcontracts are considered Direct Costs for the prime applicant. Therefore, for the parent budget, line F.5 (Subawards/Consortium/Contractual Costs) must reflect the total costs for all subcontracts. NIH continues to have a policy that excludes the consortium F&A costs from any direct cost limit. Our systems will do this calculation for us.
- An applicant may see both detailed and modular budget component
options as part of the SF424 (R&R) application package. Which should
be used?
The rules are the same as those for paper applications. If an application meets the modular limit of $250K or under, the applicant must submit a modular budget. Likewise, if an application exceeds $250K, it must come in as a detailed budget. The applicant should complete the budget component appropriate to their situation.
In 2005, NIH removed modular budgets for the SBIR/STTR applications. Therefore, SBIR/STTR applications will have only a detailed budget in the application package.
- The R&R cumulative budget page is 'automatically' filled-in
by the system – correct?
The cumulative budget is system-generated and PIs will see it as part of the R&R detailed budget component or a modular component. They do not have to do any data entry.
- On the page for the Research & Related Budget, Sections F-K,
Budget Period 1, there is a box in the upper right hand corner that states
‘Next Period.’ However, it is grayed out and we cannot access
the next period forms. How does one navigate to the screens for the next
budget period?
You must complete all the required information (i.e., those fields that are highlighted and noted with an "*") on this page before the "Next Period" button is activated.
Person Months
- How should we list partial months? In the budget period on the
SF424 (R&R) application, the number of calendar months that a senior
key person worked on a project may be listed as between 1 and 12 months.
However, some of our key personnel are only putting in half a month effort
toward the project. How do we enter that information in the budget period?
Do we change the 1/2 month to 1 month without changing the requested salary
or do we need to adjust our entire budget so everyone on the project team
puts forth more than 160 hours?
Originally the eRA Commons system only allowed whole numbers 1-12 for the number of calendar months that a senior key person worked on a project. The system has been fixed to accommodate partial months up to two decimal places (e.g., 2.55), but still has an issue with partial months less than one (e.g., .65). <Workaround> The temporary solution while eRA Commons works to fix the issue is to change the calendar months by rounding up to 1 and leave the budget unchanged. The budget justification should explain the actual effort/person months.
- How will administrative supplements be handled?
The current practice for administrative supplements will not be changed at this time. They will continue to be handled by the individual Institutes and Centers (ICs).
- Is there a limit on application files size?
Neither Grants.gov nor NIH has set limits for application size. NIH has successfully tested applications up to 140 MB. However, based on an analysis of current paper application size and experience gained from grant programs that have already transitioned to electronic submission, NIH expects an average R01 application to be in the 6-10 MB range with 99% of applications falling under 40 MB. If your application has grown substantially larger than these ranges and you are having difficulty working with the application due to its unwieldy size, here are some tips for keeping file size under control: - Have you identified the PDF attachment(s) that are contributing to the large application package size?
- Have you followed application guide instructions regarding images?
- Have you included scanned documents in your application?
- Have you followed the most recent Appendix guidelines (NOT-OD-07-018)?
- Have you included audio or video in your application?
Since the bulk of application content is contained in PDF attachments, you should check the size of each PDF file to determine which file(s) are the biggest contributors to the overall application file size. The file size is easily determined by looking at a detailed view of the local or shared directory that contains your PDF files. Once you have identified the files that may need some attention, the remaining tips may help reduce the actual file size.
The Research Plan Component section of the application guide provides the following guidance: Full-sized glossy photographs of material such as electron micrographs or gels must only be included within the page limitations of the Research Plan. The maximum size of images to be included should be approximately 1200 x 1500 pixels using 256 colors. This size is consistent with current publication standards. Figures must be readable as printed on an 8.5 x 11 inch page at normal (100%) scale. Investigators must use image compression such as JPEG or PMG. Do not include figures or photographs as separate attachments either in the Appendix or elsewhere in the application.
It is recommended that applicants avoid scanning text documents to produce the required PDFs. Instead, NIH recommends producing the documents electronically using text or word-processing software and then converting documents to PDF. We realize there are times when including scanned documents is unavoidable, but make it the exception rather than the rule when preparing your application.
The new appendix guidelines that took effect on January 3, 2007 outline the types and format of materials that are acceptable.
Although it is technically possible to include audio or video within your application, it is preferred that you work directly with your Scientific Review Administrator (SRA) after application submission. There is no guarantee that the audio or video clips will play for reviewers from within your application. By working with your SRA, you can confirm that the audio or video are allowed and guarantee the appropriate guidance can be provided to reviewers to ensure they have the software needed to play the clip.
NOTE: If you have difficulty submitting your application and believe application size may be an issue, please open a ticket with the eRA Helpdesk at http://ithelpdesk.nih.gov/eRA/. Helpdesk staff will escalate the issue to appropriate technical resources and can work with you to get your application submitted.
Attachments/Appendix
- There are a number of places where an attachment is uploaded.
What type of attachments will NIH accept?
NIH application submissions will accept only PDF attachments. Users will find a variety of information on tools and software that can be used to generate PDF attachments on Grants.gov’s Software webpage (http://www.grants.gov/agencies/asoftware.jsp) under the header ‘Convert Documents to PDF’ (http://www.grants.gov/agencies/asoftware.jsp#3).
- How will appendix material be accommodated?
There is an attachment upload available for Appendix material. Up to 10 separate PDF attachments can be included. The appendix attachment upload feature is Item 15 in the PHS 398 Research Plan Component.
- Scientific Review Administrators currently assess appendix material
for appropriateness. Will this business practice be altered?
This business practice will not be altered at this time.
- How will administrative supplements be handled?
The current practice for administrative supplements will not be changed at this time. They will continue to be handled by the individual Institutes and Centers (ICs).
- How will supplemental/additional/correction material submitted
after application submission be accommodated?
The current practice will not be altered at this time. This supplemental/additional/correction material may only be submitted with the permission of the assigned Scientific Review Administrator (SRA), and the submission is made directly to the SRA.
- Will applicants be permitted to submit supplemental/additional/correction
material without SRA permission?
At this time the process for submitting supplemental material will continue to be at the discretion of the SRA, and directly to the SRA.
- How does an applicant submit appendix material that cannot be
transmitted electronically?
"Hard" appendix materials like a video or heart valve have to be physically sent to the Scientific Review Administrator and then to the reviewers.
PDFs
- Will PIs have to generate the PDFs?
Responsibility for generating the PDF attachments depends on the business rules of the applicant organization. In most cases, the PI is given that responsibility.
- How do I avoid PDF problems?
To avoid PDF problems, keep these guidelines in mind:
- NIH only accepts attachments in PureEdge or PDF format. Do not submit
attachments in other formats such as Microsoft Word, Word Perfect, etc.
Other formats may be allowed through Grants.gov but are not accepted
by NIH.
- It is recommended that applicants avoid scanning text documents to produce the required PDFs whenever possible. Instead, NIH recommends producing the documents electronically using text or word-processing software and then converting documents to PDF. Scanning paper documents, without the proper Optical Character Recognition (OCR) process, will hamper automated processing of your application for NIH analysis and reporting. For additional information on PDF conversion software, visit the Software webpage on Grants.gov website and click on the header ‘Convert Documents to PDF’ (http://www.grants.gov/agencies/software.jsp#3).
- A 0 byte attachment is an invalid PDF.
- Only use standard characters in file names:
A through Z, a through z, and 0 through 9, Hyphen (-), underscore ( _ ).
- Disable all security features in the PDF document.
Protected documents prevent NIH from opening and processing the document. Security settings vary by PDF tool, but please ensure security settings are not marked. The applicant needs to look at the Document Security tab under Document Properties (directly from the tab) and set the security parameters to ensure open access so NIH can process the content. For instance, do not password protect the document and do not mark Content Extraction or Copying; Document Assembly, etc as “Not Allowed.”
- If you are having trouble fixing the PDF settings, simply cut and
paste from the PDF document into a Microsoft Word document and then
reconvert (in some cases it may be better to use another PDF converter).
- One of the PDF tools that have been working without issue for most
applicants is CutePDF.
Note: NIH had previously suggested that applicants not use active links in PDFs. NIH has since addressed the issue and applicants can now include active links in PDFs.
- Do not include any information in the header or footer area of the attachments. A header will be system-generated that references the name of the PD/PI. Page numbers for the footer will be system-generated in the complete application, with all pages sequentially numbered. Applicants are encouraged to use Section Headings within the document.
- NIH only accepts attachments in PureEdge or PDF format. Do not submit
attachments in other formats such as Microsoft Word, Word Perfect, etc.
Other formats may be allowed through Grants.gov but are not accepted
by NIH.
Multiple PI
- I have heard that NIH is planning to formally allow more than
one Principal Investigator — i.e., Multiple PIs — to be recognized
on an individual grant application. When will Multiple PIs be allowed on
a grant application submitted electronically?
Beginning with research grant applications submitted for February 2007 receipt dates, the NIH will allow applicants and their institutions to identify more than one Principal Investigator (PI). The Multiple PI option will be extended to most research grant applications submitted electronically through Grants.gov (http://www.grants.gov/) using the SF424 R&R application package. Grant applications that will accommodate more than one PI beginning in February include: R01, R03, R13/U13, R15, R18/U18, R21, R21/R33, R25, R33, R34, R41, R42, R43, R44, and C06/UC6 (see http://era.nih.gov/ElectronicReceipt/strategy_timeline.htm). Some types of applications including individual career awards (K08, K23, etc.), individual fellowships (F31, F32, etc.), Dissertation Grants (R36), Director’s Pioneer Awards (DP1), and Shared Instrumentation Grants (S10) will not accommodate more than a single PI. The restriction to a single PI will be described in announcements for those programs.The NIH will extend the multiple PI option to most research grant applications when they transition to an electronic format. Some paper applications submitted on PHS 398 application forms also will allow inclusion of more than one PI, but only when the multiple PI option is clearly specified in the soliciting Request for Applications (RFA) or Program Announcement (PA). Other paper applications listing more than one PI may be delayed in the review process or returned to the applicant.
The decision to apply for a single PI or a multiple PI grant will be the responsibility of the investigators and the applicant organization. Those decisions should be consistent with and justified by the scientific goals of the project. As described on the Multiple Principal Investigator website at http://grants.nih.gov/grants/multi_pi/, the NIH expects the availability of the Multiple PI option to encourage interdisciplinary and other team approaches to biomedical research.
For updates, please visit the Multiple PI Website at http://grants.nih.gov/grants/multi_pi/.
Page Limitations
- Will mechanism-specific instructions like page limitations still
apply?
Yes, page limits still will apply and some will be system enforced. For example, the system will check the combined page limit for the Specific Aims, Background and Significance, Preliminary Studies/Progress Reports and Research Design Methods sections but will not enforce the recommendations provided in the Application Guide for each of these subsection (e.g., Background and Significance should be 6 to 8 pages). Note that while these computer validations will help minimize incomplete and/or non-compliant applications, they do not replace the validations conducted by NIH staff. Applications found not to comply with the requirements may be delayed in the review process.
- What happens to page limits if the formatting changes when a PDF is generated?
NIH validations include checks for page limits. Some accommodation will be made for sections that when combined must fit within a specified limitation. While each section of the Research Plan needs to eventually be uploaded separately, applicants are encouraged to construct the Research Plan as a single document, separating sections into distinct PDF attachments just before uploading the files. In this way the applicant can better monitor formatting requirements such as page limits. When validating for page limits, the eRA Commons will not count the white space created by breaking the text into separate files for uploading. Applicants may receive a warning message similar to the following: “The Research Plan is limited to 25 pages. This may span 28 pages due to page breaks. If the total space occupied by text does not exceed 25 pages then no action is needed.” If you provide 26-28 pages of text, the system will allow the application to proceed, but remember the application is subject to further conformance checking by NIH staff.
- Is there a character limit on organization names in the R&R Budget form and the R&R Senior/Key Person Profile (Expanded) forms?
Yes. You can only use 60 or less characters in the following fields:
- 'Enter name of Organization' field on the Research & Related Budget - Section A & B form
- 'Organization Name' field on the Research & Related Senior/Key Person (Expanded) form
There is an anomaly in the PureEdge forms that allows you to enter up to 75 characters but in fact, it gets cut to 60 characters and this causes processing delays during validations at Grants.gov.
Cover Letter
- Will applicants still have the opportunity to include a cover
letter?
Yes. One of the PHS398 optional components is the Cover Letter. If multiple application submissions are necessary to correct errors, only the last cover letter submitted will be retained in the system.
- Will the cover letter include all the information currently allowed?
Yes. The instructions for the cover letter remain the same. At this time it will be a PDF upload of the relevant information, not structured data.
Application checks (Validations)
- Can you explain the differences in the checks that Grants.gov
does on the application and those done by NIH?
The Grants.gov validations are minor and straightforward - things like checking to make sure no viruses are attached to the application and checking to ensure the DUNS number is correct. At the NIH level, the application is checked against business rules - such as whether you have an assurance number if the human subjects is marked "yes".
- Could you detail what will be the validations (business rules)
that an application will be checked for - such as page limits?
The list of errors and warnings that an applicant may encounter during the validation process, along with tips to help you understand these better, are available on the Prepare Application page.
Submit Your Application
Submission Deadline
- What is the submission deadline — the date/time the application
is stamped as received by Grants.gov or the date/time the data is received
by NIH?
Applications must be submitted to Grants.gov by 5 p.m. local time (of the applicant institution/organization) on the submission/receipt date. If the submission/receipt date falls on a weekend or Federal holiday, the date will be extended to the next business day.
- What is on-time submission?
- For an application to be on time, ultimately it will need to be a completely error-free application (i.e., passed Grants.gov and eRA Commons validations without errors) submitted to Grants.gov by 5:00 p.m. local time of the applicant organization on the receipt date. If the submission deadline falls on a weekend or Federal holiday, the date will be extended to the next business day.
- However, during the transition from paper to electronic applications, NIH is allowing a correction window of five business days to help applicant organizations adjust to the new process. NIH expects that all registration requirements are met prior to the initial application submission and that the initial application is submitted to Grants.gov on or before the submission deadline. Further, any errors must be corrected and an application image generated within the five business days following the submission deadline.
- This five business day correction window is for correcting errors and/or warnings that were identified by the electronic eRA system. This window is not for tweaking the content of the research plan or other parts of the application, but for working through specific errors on the forms identified during the submission process.
- Please remember that NIH will not maintain this correction window for the long term. NIH strongly recommends that applicant organizations target an error-free application to Grants.gov by the deadline now, so that when the correction window shrinks or goes away, they will be prepared.
- Note: If the correction window of five business days is used to correct an application after the receipt deadline, the application must include a cover letter including the Grants.gov tracking number for the original submission and an explanation for why the corrected application is required. If a cover letter is already included with the application, this information should be added to the original cover letter. Use the PHS 398 Cover Letter form component found with the optional documents in the application package to attach a cover letter to the application.
- What is NIH's policy for late applications?
The late policy remains in effect regardless of mode of delivery (paper or electronic). See the following NIH Guide Notices:- NIH Policy on Late Submission of Grant Applications (Jan. 4, 2008)
- Clarification
of NIH Policy on Late Submission of Grant Applications (Sept.
2, 2008)
System problems
- What contingency plans are in place in case the Grants.gov and
eRA Commons systems have technical problems on a submission/receipt date?
If an application has to be submitted again because of Grants.gov system
problems, will these be considered "late"?
NIH will not penalize the applicant for Grants.gov or eRA Commons system issues. If you encounter a system problem, immediately contact the eRA Commons Help Desk to report the issue. As soon as the help desk staff confirms a system issue, they will document the issue and continue to work with you until the problem is resolved.NIH's contingencies provide for extending submission dates when Grants.gov is unavailable for a significant period of time leading up to a deadline. The NIH Guide, Electronic Submission Program email lists and Electronic Submission Web site will be the primary vehicles used to communicate any deadline extensions. If eRA Commons experiences a significant interruption in service just after a submission deadline, the error-correction window may be extended to provide applicants with the necessary time to check submission status, address errors and view their corrected applications. The Electronic Submission email lists and Electronic Submission website will be the primary vehicle used to communicate error-correction window extensions.
Please be aware that if your submission failed to complete because you did not follow all the instructions, NIH is under no obligation to accept your late application.
Check Submission Status
Status
- How can a PI or a Signing Official track their application?
Authorized Organizational Representatives can track their applications through Grants.gov. The AOR also gets an email confirmation both when Grants.gov receives the application and when it passes to NIH. After the application is processed by eRA Commons, the PI or SO can log in to the eRA Commons and view the status of their application. eRA Commons sends email notifications to both the AOR/SO and PI at different stages of processing. Note: It is the applicants' responsibility to track their application through Grants.gov and NIH eRA Commons
- I submitted my application to Grants.gov but cannot see any status
regarding the application within the eRA Commons.
If you cannot see the status of your application, it may be due to one of these two reasons:
- The application did not contain a valid Project Director/Principal
Investigator (PD/PI) eRA Commons user ID. This field is not marked as
required on the government-wide form, but it is required by NIH.
Action: Check the 'Credential, e.g., agency login:' field in the 'Profile - Project Director/Principal Investigator' section of the Senior/Key Person Profile(s) component of your application to ensure a valid PD/PI eRA Commons user ID was included and entered in all capital letters. It is important to include the PD/PI user ID and not the Signing Official (SO) user ID in this field. You will need to submit the corrected application through Grants.gov in order to view application status in the eRA Commons.
Be sure to check the Changed/Corrected application box in the Type of Submission field of the SF 424 (R&R) cover component. Once that box is checked you will notice that Grants.gov will require data in the Federal Identifier field. If you are submitting a new project application (including corrected submissions for new applications) simply enter “N/A” in this field. For a continuation, revision, or renewal application, enter the assigned Federal Identifier number or award number (e.g., 1 R01 CA 123456-01).
- NIH has not yet processed the application.
Action: If the AOR has not yet received an email notification from Grants.gov that the grantor agency has retrieved the application, the AOR should work with Grants.gov to check on application status. Otherwise, continue to periodically check the eRA Commons for application status. Although applications are typically processed in a matter of hours, Grants.gov may take up to 48 hours and eRA Commons an additional business day to process an application.
- The application did not contain a valid Project Director/Principal
Investigator (PD/PI) eRA Commons user ID. This field is not marked as
required on the government-wide form, but it is required by NIH.
- Will applicant organizations have a chance to take a look at
the data once NIH has received it?
Yes. After NIH receives an error-free application package, it assembles the final application the way a reviewer would see it. The PI and SO have two full weekdays (Monday - Friday, excluded Federal holidays) to view the application after which the submission process is complete and the application moves forward to NIH Receipt and Referral.
Email Notifications
- What kind of email notifications are sent to applicants by Grants.gov
and by eRA Commons during the submission process?
A detailed listing of the email notifications sent by both Grants.gov and eRA Commons can be found at Chart of Email Notifications.
- I am an AOR/SO and I have received my email notification from
Grants.gov but have not received any notifications from eRA Commons indicating
the application has been processed. What do I do?
Since email can be unreliable, it is the applicant’s responsibility to periodically check the eRA Commons for the status of these applications. If you do not receive any notifications from eRA Commons and do not see the application status in eRA Commons after two days, contact the eRA Commons Helpdesk.
Submitting Changed/Corrected Applications
- How are changes or corrections to applications submitted?
It is sometimes necessary to submit changes or corrections to an application submission. For example, if any Errors are identified in a submission they must be corrected and the entire application submitted again in order to complete the process.
Here are the steps to submit a corrected application:
- Make the corrections to the application forms.
- On the first page of the SF424 (R&R) application form, check the
Changed/Corrected application box in the Type of Submission field located
in box 1.
- Once the Changed/Corrected box is checked, box 4, the Federal Identifier,
becomes a required field. If you are submitting a “New” project
application (including corrected submissions for “New” applications),
enter the Grants.gov tracking number of the previous submission attempt
(e.g. GRANT00123456). If you are unable to find the tracking number,
enter “N/A”. For “Renewal”, “Resubmission” or “Revision” applications,
the Federal Identifier field should contain the IC and serial number
of the prior application/award number (e.g. CA123456).
- If after the submission deadline, include a cover letter with an explanation
of your changes and why the application is late. Include information
from any previous cover letter(s) since that information is not retained.
The cover letter must be in PDF format and attached to the PHS Cover
Letter component of the application.
- The entire Changed/Corrected application must be submitted through
Grants.gov.
- Make the corrections to the application forms.
- Is a cover letter required with Changed/Corrected applications?
Changed/Corrected applications submitted before the submission date do not require a cover letter. Any application submitted after the submission date (even if within the five business day correction window that NIH is allowing as we transition) must include a cover letter. The cover letter is not saved from one application submission attempt to the next, so the cover letter submitted with the final assembled application should include ALL the information that you want to convey to NIH. For electronic submissions, the cover letter is an attachment (in PDF format) to the PHS 398 Cover Letter File component found in the Optional Documents section of the application package.
- If the original application came in on the PHS398, how does
a resubmission come in?
Once a grant program/mechanism transitions to electronic submission, all new, renewal, resubmission or revision applications must use the electronic SF 424 (R&R) application (even if the original application was submitted in paper PHS 398 format).
- Can I still view and correct an application after the application due date?
NIH sets the application due date published in the funding opportunity announcements (FOAs) the same as the last receipt date for all FOAs. Unlike other agencies, NIH may accept applications that are submitted after the due date to accommodate our correction window, viewing window and late policy should it apply.
Check Assembled Application
- When will the application move forward for processing?
After two full weekdays (Monday-Friday, excludes Federal holidays), if not explicitly rejected by the AOR/SO, the application will automatically move forward to the Division of Receipt and Referral for processing. For example, if you submit your application on Monday then you have all day Tuesday and Wednesday to view the application. The application will move forward at 12:01 a.m. ET on Thursday morning.
- Will the AOR/SO and PD/PI be notified to check the assembled application?
Yes, both the AOR/SO and the PD/PI will be sent an email notification to check the application for completeness. A notification also is sent if the AOR/SO rejects the application.
- Can the AOR/SO “Reject” the application if the two
day window falls after the submission deadline?
Only the AOR/SO has the ability to "reject" the application within the two weekdays viewing window:
- to address warnings or
- if the assembled application in eRA Commons does not correctly reflect the submitted application package due to system issues with eRA Commons or Grants.gov (i.e. some part of the application was lost during the submission process or did not transfer correctly).
- Can the PD/PI have the option to “Reject” the application?
No. The PD/PI must work through the AOR/SO to “Reject” an application.
- Are holidays included in the two weekday period?
Federal holidays are excluded in the two weekday period. For example, if July 4 (Independence Day) falls during the two weekday period, it is not counted towards the two weekdays.
- What can I do if I find a problem after the application moves
forward for processing?
You should contact the Scientific Review Administrator assigned to your application for advice and guidance. Once the application moves to the Division of Receipt and Referral, the helpdesk staff can no longer assist with changes to the application.
- The bookmarks in my assembled application in eRA Commons do not
work as expected; I am sometimes sent to the incorrect page. What should
I do?
This is an issue that some users have run into, especially if using Adobe Acrobat Professional. The control icons at the bottom of the Adobe display allow you to set the viewing mode to Single Page, Continuous, Continuous Facing or Facing. The default, Single Page, is the viewing mode that has given users trouble. You can click on the icons to change the viewing mode setting. We suggest using Continuous mode (just to the right of Single page mode; the Continuous mode icon shows one page on top of the other).
Special Categories
Adobe SF424 (R&R) Forms
- When will NIH begin using the new Adobe forms?
NIH began to pilot the Adobe forms in the fall of 2008 with a few select funding opportunity announcements. The first standing submission deadlines using Adobe forms will be in January 2009. Additional information about the transition can be found by viewing the NIH Resources for the Adobe Transition page.
- What changes should users expect? Grants.gov is phasing out PureEdge based applications and users will be
required to use new Adobe based forms. The basic look and feel of the
forms remains the same. The overall process of finding opportunities,
downloading application packages, preparing forms and submitting applications
will also remain the same. Users will notice some small differences in
form layout, but the main differences are in form navigation.
- How do I move my data from a PureEdge form to an Adobe form?
Unfortunately there is no automated way to extract information from a PureEdge form to populate the Adobe application. Users will need to manually re-enter their data by either cutting and pasting or re-typing into the new form.
- What software do I need to complete the new forms?
As of November 17, 2008, Grants.gov recommends Adobe Reader version 8.1.3 or higher to complete the new forms. Users should regularly check the Grants.gov Download Software page for updates or changes, and for information on how to download the Grants.gov compatible Adobe reader for free.
- What software do I need to create PDF attachments?
Applicants may use one of the free PDF Conversion Programs recommended by Grants.gov or Adobe Acrobat (Standard or Professional) to create the required PDF attachments
- Are the Adobe forms compatible
with both Mac and PC systems?
Yes, the new Adobe forms offer platform independence and work with both Mac and PC operating systems.
- How do I get support for the new Adobe forms?
Applicants should follow our usual process for seeking support. Any questions on form navigation, functionality or submission of the forms to Grants.gov should be directed to the Grants.gov Contact Center. If you encounter a technical issue that threatens NIH’s timely receipt of the application, please contact the eRA Help Desk at NIH to document the issue and provide us with the Grants.gov Contact Center tracking number.
- Where can I go to find more information about Adobe software?
For specific software related questions and more information about using Adobe programs to complete your application, please visit the Adobe Reader section of Grant.gov’s FAQs. These FAQs provide guidance on Adobe topics such as:
- Understanding Adobe error messages
- Using compatible versions of Adobe Acrobat to complete your application
- How to work with multiple Adobe programs (Reader and Acrobat)
- Setting Adobe Reader as your default application viewer
- Updating your Adobe software
- Understanding Adobe error messages
NIH & Grants.gov
NIH
- Why is NIH embracing electronic submission and what are the benefits
of integrating with Grants.gov's centralized website?
The impetus behind this change is the Federal Financial Assistance Management Improvement Act of 1999 (Public Law 106-107) and the President's Management Agenda that is driving Federal Agencies to simplify Federal financial assistance application requirements and create a single website to apply for Federal assistance. Grants.gov (http://www.grants.gov/) has been designated by the Office of Management and Budget as the single access point for all grant programs offered by 26 Federal grant-making agencies. NIH's Electronic Receipt program and integration with Grants.gov is designed to give applicants a convenient one-stop shop for finding and applying for grant applications. The goal is also to provide a consistent look and feel to grant applications across federal agencies.
- What is NIH's electronic submission goal?
By the end of September 2007, NIH plans to:
- Transition from the Public Health Service 398 (PHS398) to the new
federal government-wide Standard Form 424 Research & Related (SF424
(R&R)) dataset and the SF424 Discretionary dataset. Note: SF424
Discretionary will be of limited use for NIH.
- Simultaneously transition to electronic submission through the federal
government-wide Grants.gov "Find and Apply" website.
- Achieve the Office of Management and Budget goal of posting 75 percent
of its Funding Opportunities in Grants.gov’s “Find”
section an Grants.gov’s “Apply” section.
- Transition from the Public Health Service 398 (PHS398) to the new
federal government-wide Standard Form 424 Research & Related (SF424
(R&R)) dataset and the SF424 Discretionary dataset. Note: SF424
Discretionary will be of limited use for NIH.
- What are the expected benefits of electronic submission?
Benefits of electronic submission include:
- Facilitates efficiencies to shorten the cycle from application receipt to award
- Provides clear, color grant image
- Creates a comprehensive repository of data that can be mined by knowledge management and other portfolio tools
- Improves data quality (e.g., enforcement of business validations)
- Saves >200,000,000 pieces of paper/year (estimated) and countless hours of human effort
- Reduces scanning, printing and data-entry costs
- What is NIH's role once the applications are submitted to Grants.gov?
NIH will retrieve applications from Grants.gov in a system-to-system mode. NIH then will check the application to ensure that all agency-specific guidelines have been followed.
If the application has errors that make it unacceptable, NIH will send a message to the applicant to check eRA Commons for errors. The applicant then will have the opportunity to correct the application and submit the entire application again via Grants.gov.
If there are no errors, NIH will notify the Principal Investigator and Signing Official (SO) to go to eRA Commons to check the assembled application. The SO will have the option to “reject” the application if the assembled application does not correctly reflect their submission due to system issues. If no action is taken, the application will move to the referral process after two full business days.
- What is Grants.gov?
Grants.gov is a cross-agency initiative spanning 900 grant programs in 26 grant-making agencies, and more than $350 billion in annual awards. It provides a single source to find Federal government-wide competitive grant opportunities. It is the Federal government's single, online portal for any person, business, or State, Local and Tribal government to electronically find grant opportunities and apply for grants.
- What functionality does Grants.gov provide?
Grants.gov provides robust functionality for the grant community, including: - "Find Grant Opportunities" allows users to search for available grant opportunities and register to receive notification of grant opportunities.
- "Apply for Grants" allows users to search for and download application packages, complete application packages offline, submit completed application packages and track the status of submitted applications.
- When will applicants be able to submit grant applications electronically
to NIH via the Grants.gov portal?
NIH began the transition to electronic submission in December 2005 when it chose the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grant applications as the first grant type for applicants to submit electronically to NIH via Grants.gov.
NIH is transitioning to electronic submission by individual research program/funding mechanism. Funding Opportunity Announcements (also known as Request-for-Applications and Program Announcements) will be issued in the NIH Guide and posted in Grants.gov as mechanisms are transitioned.
See also: NIH Transition Timeline
- Does Grants.gov architecture have sufficient capacity to handle
receipt of grant applications from the entire Federal government?
In the past two years, Grants.gov has substantially increased its capacity to handle submissions from an initial 500 submissions a day to 4,000 submissions a day. Grant.gov’s Apply functionality was launched Oct. 31, 2003, with four servers; recent infrastructure upgrades carried out in November 2005 have more than tripled the number of servers to 13.
Grants.gov is committed to ensuring that it meets the needs of its customers. Towards this end, it is finalizing a long-term architecture plan that will incrementally scale up its architecture for increased capacity to keep several steps ahead of the surging usage of its system across the grantee community and the federal grant-making agencies. Grants.gov is scheduling its next infrastructure upgrade for Spring 2006, timing it just before it receives its heaviest application load of the fiscal year.
Grants.gov is also working with the federal grant-making agencies to stagger submission deadlines to avoid overwhelming system usage on any given day. Applicants are encouraged to apply at least 24 hours prior to the deadline date to minimize concerns related to site capacity.
SBIR/STTR
Registration
- Is Your Small Business and is the Project Director/Principal Investigator
(PI) Registered with eRA Commons?
In addition to registering with Grants.gov, both the small business concern and the Project Director/Principal Investigator (PD/PI) must also complete a one-time registration in the eRA Commons in order to submit applications to NIH. Organizational officials are responsible for registering the PD/PI in the eRA Commons. To find out if your organization is already Commons-registered, a "List of Commons Registered Organizations" can be found at: http://era.nih.gov/userreports/ipf_com_org_list.cfm.
The PD/PI registration must be done by an organization official or their delegate who is already registered in the Commons. The PD/PI should work with their Authorized Organizational Representative (also known as Signing Official in the eRA Commons) to determine their institutional process for registration. For a step-by-step account, click on Grantee Registration Steps for Commons (PDF - 37 KB). (Note: This applies to applicants as well as grantees despite the reference to “Grantee”.)
The eRA Commons is so important because this is now THE discrete information exchange system where NIH and the applicant/grantee community are able conduct their extramural research administration business electronically. As announced in a recent NIH Guide Notice, the following changes are currently in place:In order to avoid delays in the e-notification process, it is vital that all Applicant/Grantee Organizations, Principal lnvestigators are registered in the eRA Commons and e-mail addresses are checked periodically for accuracy.
Special Note for STTR applicants: The STTR applicant organization must officially affiliate the PD/PI with the small business concern in the Commons if the PD/PI is not an employee of the small business concern.
Following are the steps to affiliate a PD/PI to the applicant organization/institution:- PD/PI gives Commons user ID and email address to the administrator of the applicant institution. (The email address must be the one that is contained in the Personal Profile for the PI.)
- Administrator logs into the Commons. (The administrator can be the Signing Official, Administrative Official, or the Accounts Administrator.)
- Administrator selects "Administration" tab and then "Accounts" tab.
- Administrator selects "Create Affiliation" tab.
- Administrator enters the Commons User ID and Email address into the appropriate fields and clicks "Submit."
Note: The account cannot have any other roles attached to it other than the PD/PI.
- May I still use the Omnibus Solicitation of the NIH, CDC, and
FDA for SBIR/STTR Grant Applications or must I find an Institute or Center
(IC)- specific FOA (i.e., Program Announcement [PA] or Request for Application
[RFA])?
In http://grants.gov/search/searchHome.do, a Parent SBIR FOA (PA-06-120) http://grants.nih.gov/grants/guide/pa-files/PA-06-120.html and a separate Parent STTR FOA (PA-06-121) http://grants.nih.gov/grants/guide/pa-files/PA-06-121.html have been issued to encompass the general scientific research areas that are described in the 2006 SBIR and STTR Omnibus Solicitations (NIH, CDC, and FDA) Program Descriptions and Research Topics section of the Omnibus Solicitation. See http://grants.nih.gov/grants/funding/sbirsttr1/2006-2_SBIR-STTR-topics.doc (MS Word - 1.40 MB).
To apply for these opportunities, you must use the parent FOA number (i.e., PA-06-120 or PA-06-121). In short, think of the “Parent” announcements as the “omnibus” solicitation now.
While the Parent SBIR and STTR FOAs encompass all 24 ICs’ research topics and those of the CDC and FDA, there are other specific SBIR and STTR FOAs in Grants.gov that you may still apply for. Some NIH awarding components (e.g., National Cancer Institute, National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Digestive and Kidney Diseases) have identified additional specific SBIR and STTR funding opportunities, and these are also available in http://grants.gov/search/searchHome.do (or http://grants.gov/Apply if the FOA number is known). For a full list that includes FOA numbers, see the NIH Special Announcements for Small Business Research Opportunities Web site.
- How Do I Find and Apply for an SBIR/STTR Funding Opportunity?
A new advanced search feature for the NIH Guide for Grants and Contracts (http://grants.nih.gov/grants/guide/search_guide.htm) allows the user to search by application package (i.e. PHS 398 or SF424).
A button added to the Guide announcements allows the applicant to access the Grants.gov application package directly from the Guide.
Once you identify a grant opportunity at Grants.gov, follow the steps described at http://era.nih.gov/ElectronicReceipt/applying.cfm to submit an application. You must download the SF424 (R&R) Application Package and Application Guide (MS Word - 24 KB) for a specific FOA (e.g., PA-06-120, PA-06-121) through the Grants.gov Apply Web site. Only the forms package directly attached to a specific FOA may be used.
Remember: Before you can apply electronically, the applicant small business organization must be registered in Grants.gov and the eRA Commons.
- In the SBIR/STTR application package (PA
06-120) that I downloaded from Grants.gov, the field of Opportunity
Close Date is prepopulated with the closing date of Jan. 3, 2007. However,
the Small Business Funding Opportunities webpage (http://grants.nih.gov/grants/funding/sbirsttr_receipt_dates.htm)
lists April 1, 2006 as the submission date. Which is the correct closing
date?
A “closing” date is equivalent to the “expiration” date of a FOA. The closing date is Jan. 3, 2007 for this funding opportunity.
The “closing/expiration” date is different from the submission date. The submission date reflects the deadline for your application to be received by NIH; that date is April 1, 2006 for this application package.
In reading any Funding Opportunity Announcement (FOA) in the NIH Guide for Grants and Contracts:
- A posted date refers to the date the FOA is posted on Grants.gov Apply. An applicant can download the application package on that date and begin filling it out. However, the applicant has to wait until the FOA’s opening date to submit the application.
- An application can be submitted anytime between the opening date and the application submission date noted for AIDS and non-AIDS applications (Standard dates may apply; check http://grants.nih.gov/grants/funding/submissionschedule.htm for details)
- When you download an application package from Grants.gov, the expiration
date is prepopulated. Do not go strictly by this date since it may not
apply to your particular situation; for instance, it may reflect the submission
date for AIDS applications and you may be submitting a non-AIDS applications
that is due earlier. In this case, the prepopulated date has no bearing
on your application and you need not be concerned by it.
- Why Are There Separate FOAs for the SBIR and STTR Grant Mechanisms?
Each FOA is linked to a specific Application Package. Since STTR applications have slightly different forms requirements than SBIR applications, distinct application packages had to be developed for each of these mechanisms. The good news is that you will know exactly which forms to include if you are applying for an SBIR award and which to include if you are applying for an STTR award since they will be pre-packaged.
Note: In most cases, you will find parallel FOAs of identical scientific scope (e.g., one that utilizes the SBIR (R43/R44) grant mechanisms and a parallel one that utilizes the STTR (R41/R42) grant mechanisms. We have clearly distinguished the mechanisms in the FOA title, and we also include a cross-link to the parallel FOA. Applicants may not simultaneously submit identical/essentially identical applications under two HHS funding opportunities, including the SBIR or STTR Parent FOAs (PA-06-120 or PA-06-121). - If a Grantee Organization with the same PI wants to submit two
totally separate applications under the same Funding Opportunity Announcement
for the same submission date, will the validations in electronic submission
allow this? Or will the system see one as merely a replacement of the other
and essentially overwrite the previous application?
The validations look for a combination of three things —Commons Account Name for the PI, Title, and Council Round. If all three are a match to an existing record, and "corrected/changed application" is not checked, then the applicant will receive an "error" e-mail.
However, as long as the title is distinct, multiple applications from the same PI, for the same submission date should not be a problem.
- An answer of "yes" to Question 8 of the SBIR/STTR Information
component "Have you received SBIR Phase II awards from the Federal
Government" requires an attachment explaining my company's commercialization
history. How do I answer the question if I have less than 15 Phase II SBIR
awards since a commercialization history is then not to be submitted?
If you have received SBIR Phase II awards from the Federal Government (including NIH), check the YES box. Attach a file that includes either: (1) a statement indicating that the applicant small business has not received more than 15 SBIR Phase II awards from the Federal Government during the preceding five fiscal years; or (2) a company commercialization history if you have received more than 15 Phase II SBIR awards from the Federal Government during the preceding five fiscal years. The history must document the extent to which the company was able to secure Phase III funding to develop concepts resulting from previous Phase II SBIR awards, and for each Phase II award the history must include: (1) name of awarding agency; (2) award number and date; (3) amount of award; (4) title of project; (5) source, date, and amount of Phase III funding agreement; and (6) commercialization status of each Phase II award.
If you have not received SBIR Phase II awards, then check the NO box.
- How does one add a key personnel that is to be hired?
A “key person” must be a named individual, since this person’s particular credentials and accomplishments are given critical consideration during the review of the application. Project roles, for which no specific person has been named, cannot be reviewed in this way and therefore should not be listed in this section.
However, TBH (to-be-hired) individuals may be listed with the R&R Budget, in section A (Senior/Key Person). On this form the emphasis is not only on named individuals, but on all senior/key positions that comprise the budget for the proposed project. It is here that key TBH positions may be listed, but you should list these positions AFTER the names, roles, months, salaries, and fringe benefits have been entered for all of the named senior/key personnel who have been listed on the “Research & Related Senior/Key Person” form.
- Is There A Dictionary to Help Me Understand This New SF424 (R&R)
Lexicon?
As we are also adapting to the new terminology, we have created the following cheat sheet, which may be helpful to you as well.
NIH Terminology SF424 (R&R) Terminology What’s “Out” What’s “In” New (T-1) New Competing Continuation (T-2) Renewal Revision or Amendment Resubmission Competing Supplement Revision Program Announcement (PA) and/or Request for Application (RFA) Funding Opportunity Announcement (FOA)—general term for all PAs and RFAs Principal Investigator (PI) Project Director/Principal Investigator (PD/PI)(Combined term) Authorized Organizational Official (AOO) or Signing Official (SO) Authorized Organizational Representative (AOR) Other Support Current & Pending Support Literature Cited
(Part G. of 398 Research Plan)“Bibliography & References Cited” (SF424 (R&R) Other Project Information Component Consortium Budget Subaward Budget
Note: SF424 (R&R): Type of Application also includes “Continuation.” This is equivalent to our Progress Report or T-5.
NIH will not use the SF424 (R&R) for Progress Reports.
- Why Can't I Enter the Entire Federal Wide Assurance (FWA) Number
in the Human Assurances Number Field?
In the past, applicants entered on the PHS 398 all eleven alpha and numeric characters (e.g., FWA00012345).
On the SF424 “RESEARCH & RELATED Other Project Information” form, use only the numeric portion of the FWA (e.g., 00012345) as this field is limited to 10 characters (i.e., drop “FWA”).
- How Do I Track Page Limitations When I Have to Submit All of the
Various Sections of the Research Plan as Separate PDF Files?
Separate attachments have been designed for the Research Plan sections to maximize automatic validations conducted by the eRA system. When the application is received by the agency, all of the Research Plan sections will be concatenated in the appropriate order so that reviewers and agency staff will see a single cohesive Research Plan.
While each section of the Research Plan needs to eventually be uploaded separately, applicants are encouraged to construct the Research Plan as a single document, separating sections into distinct PDF attachments just before uploading the files. In this way the applicant can better monitor formatting requirements such as page limits.
We suggest that you create the Research Plan in MS Word or some other word processing software; keep it to the 15 pages for Phase I, and 25 pages for Phase II Sections 2-5. Then “excise” the appropriate sections for each “Item” (2-5), and create a PDF from each excised part. There will be white space, which is fine, and expected.
- My organization is applying for a grant; however the PI on the
grant application works in a different organization. On the SF424 (R&R)
Cover component, the PI information in Item 15 reflects his organization,
which is different than the applicant organization information in Item 5.
When I go to the budget forms for period 1, the "Enter name of organization"
is already pre-filled with the PI's organization name. This field does not
allow edits; therefore I cannot change this field to reflect my organization
name. What do I do?
The Research and Related Budget component is incorrectly pre-populating the Organization Name. In order to proceed with your submission, please just ignore this field. The applicant organization should prepare its Research & Related Budget pages, making sure they indicated “Project” for the Budget Type. If a subaward/consortium is involved, the consortium grantee should complete their budget pages, making sure they indicate “Subaward/Consortium” for the Budget Type. The Budget Type field will help NIH staff properly distinguish between the 2 budgets until this glitch in the forms is fixed.
- Will paper applications be accepted for the submission deadline
of April 1, 2006?
No. As of Dec. 1, 2005, when SBIR/STTR applications were required to come in electronically via Grants.gov to NIH, no paper applications are accepted. You MUST submit the SBIR/STTR application electronically to Grants.gov using the SF424 (R&R) Application Package (attached to the specific FOA) and following the SF424 (R&R) Application Guide (MS Word - 24 KB). This includes all new as well as resubmission (formerly called amended/revised) applications.
- If all instructions for a research grant will be included in the
application guide, what happens to the specific SBIR/STTR instructions?
Will the Application Guide include the nuances of the SBIR program, or will
specific SBIR instructions remain in the SBIR solicitation? Will an applicant
need to sift through the information in the SF424 (R&R) instructions,
the agency-specific instructions, and the instructions in the solicitation?
NIH has created a separate Application Guide just for SBIR/STTR applications (http://grants.nih.gov/grants/funding/424/index.htm) that incorporates the SBIR/STTR instructions currently found in the solicitation with the standard application instructions.
- What about Change of Grantee Institution applications - which
application will NIH require grantees to use?
For now grantees will continue to submit these directly to the Institute/Center (IC) using a PHS398. However as mechanisms switch over to the SF424 (R&R), polices such as Change of Grantee Institution will also be reassessed and business process changes announced.
- Will non-competing progress reports be submitted through Grants.gov?
Not at this time. NIH grantees are reminded that the eRA Commons includes the capability to electronically submit progress reports for grants awarded under the Streamlined Non-Competing Award Process (SNAP). Information on the feature, know as "eSNAP", can be found on the eRA Commons Homepage: https://commons.era.nih.gov/commons/index.jsp
- What will happen with existing Program Announcements (PAs) and
Request for Applications (RFAs)?
As decisions are made to convert a particular mechanism to Grants.gov submissions, any and all PAs & RFAs will need to be re-written. The NIH Office of Extramural Policy (OEP) is generating new Funding Announcement templates as a tool for ICs to use.
- What planning and thoughts are being given to distinguish SBIR
submissions to grant programs versus submission to the Omnibus SBIR Contract
Solicitations?
Right now, Grants.gov only allows for grants submission. NIH will continue paper proposals for contract solicitations.
- Are their any future plans in regard to these contract solicitations
to 1) use of SF 424 format and/or 2) for acceptance and routing through
an electronic system?
We are not aware of parallel activities for contracts.
Miscellaneous
- For SBIR/STTR Fast-Track, there were previously two separate applications.
With SF424 (R&R), how are applicants being made aware that they must
submit an integrated application, and how they might best present this “story”
to the grant reviewers?
What we determined for a number of reasons is the need for applicants to basically tell a story — In Phase I we will do this, in Phase II there will be specific gains. Our SBIR/STTR application guide will advise applicants to do this. Another important change is that at the review stage, the reviewers will not have an option of decoupling the Phase I from Phase II. As a Fast-Track applicant, the research plan will have to be carefully written, clearly delineating Phase I and its milestones as well as what will be accomplished in Phase II.
- Are special trainings being offered to SBIR/STTR review groups
so that they know how to evaluate a combined application that cannot be
decoupled?
The Scientific Review Administrators will provide instructions to reviewers.
Impact on Review
- How will electronic submission impact peer review from an applicant’s
perspective?
There will be no philosophical changes to peer review of electronically submitted grant applications since the same review criteria and conflict of interest rules will be in effect. Peer review has always been science and merit driven and will continue to be so. Applicants will have the same access to the Scientific Review Administrator (SRA) of the study section to ask questions and air concerns.
However, there will be some practical changes:- A real plus is that is that there will be no loss of quality due to scanning/copying. The reviewers will see color images and high resolution graphics.
- All applications for each grant mechanism (e.g. R01 or R21) will transition
together to ensure continuity and fairness in the review process, so
that study sections are not dealing with both paper and electronic applications
for the same grant mechanism at the same time.
- How will electronic submission affect peer review from a reviewer’s
perspective?
Reviewers will continue to use the same review criteria and conflict of interest rules; therefore peer review will see no “philosophical” change. However, there will be some practical changes:- Reviewers will see high quality color images and high resolution graphics in the applications since there will be no diminution due to copying/scanning.
- The review will ultimately aim to be “paperless” with the exception of the CD and Conflict of Interest forms. For now, updates/corrections may be in paper.
- All relevant material will be available on the Internet Assisted Review web module.
- Reviewers will need to learn to navigate through the new SF424 (R&R) form, becoming familiar with the layout and location of information.
- Reviewers will have the option to print selected parts of the application (e.g. Research Plan) if necessary.
- Another plus is that system checks should lead to more complete/compliant applications — for example, if Vertebrate Animals is checked as yes, the application must have that PDF.
Tips for Reviewers - Viewing NIH Electronic Grant Applications (PDF - 54 KB) | February 12, 2007
Service Providers
- Who is a Service Provider?
A Service Provider is a commercial company that assists applicants, for a fee, in submitting grant applications electronically to NIH.
- As I understand it, there will be more than one way for applicants
to apply electronically --software that can be downloaded from Grants.gov
or Service Provider software that will be specific to each provider. True?
Yes. Additional information on participating Service Providers can be found on the main page of this website.
- Could NIH recommend a particular Service Provider?
No. NIH works closely with the Service Providers but cannot endorse any one Service Provider.
- If an applicant organization uses a Service Provider, who is responsible
for completing the application, the PI, the organization, or the Service
Provider?
The Authorized Organizational Representative is the person responsible, but how this process works within an organization and a service provider system varies.
- Where can I obtain information on service providers?
Please see the link on this website for Service Providers. NIH cannot endorse any service provider or commercial business.
- If an applicant is using a service provider, can they print out
their application or portions of their application (i.e. - the budget pages)
before submission and acceptance by Grants.gov and NIH?
It depends on the capabilities of the Service Provider. Some Service Providers do provide this capability.
System to System
- Where can I find information on the XML schema
used for system-to-system transmissions?
The development of a system-to-system interface with Grants.gov is between the applicant institution and Grants.gov. Therefore, the best source for this information is the Applicant System to System Integration webpage on the Grants.gov website (http://www.grants.gov/agencies/applicant_system_integration.jsp).
- How will System-to-System Trading Partners know
if forms changes are planned?
All of the Grants.gov form components are subject to change, including the SF424 (R&R) and agency-specific forms used by NIH (labeled PHS 398). Although NIH is an active member of the Research and Related Working Group that makes form change recommendations to Grants.gov, Grants.gov has responsibility for change management of the government-wide form components. For the PHS 398 components, NIH will have to schedule requested changes with the Grants.gov forms development team. When any such plans are made, NIH will post the pending change and approximate timing on our Electronic Submission website.
- Which form components will NIH use for its opportunities?
For each grant program (mechanism) NIH will use a combination of government-wide and agency-specific forms (PHS 398 and SBIR/STTR) listed on the Grants.gov website under SF424 R&R Family (http://apply.grants.gov/agency/FormLinks?family=3). Since NIH requires eRA Commons registration for electronic submission, the Research & Related Personal Data government-wide form will not be used by NIH.
- Each funding opportunity announcement lists both
mandatory and optional components within the application package. Can
NIH notify system-to-system providers of the mandatory and optional
components to be used for each grant program (mechanism)?
The specific mandatory and optional components have not yet been defined for all mechanisms. NIH has internal working groups that are working with the nuances of each mechanism and fitting them to the SF424 (R&R) form set. As decisions are made for individual mechanisms NIH will post this information to the Electronic Submission website. NIH will generally follow one of 5 templates based on the budget/special components used (Modular, Non-modular, both Modular and Non-modular, SBIR and STTR).
- Will NIH accept Greek characters in the XML data
streams?
No. Although XML will allow the characters, the Greek characters cause issues downstream. NIH's current data model and presentation interfaces are not set up to handle these characters.
- Will a System-to-System Trading Partner need to
accommodate different versions of application forms?
Yes. A System-to-System Trading Partner will need to have the flexibility to develop and maintain multiple schemas in order to support different versions of forms. Remember that some NIH funding opportunities stay in place for up to three years. NIH will not always go back to previously posted opportunities to have them pick up the latest forms, so System-to-System developers will want to be able to support either form - the original and the latest form. NIH is currently developing the ability to handle multiple schemas on the agency side.
- If a System-to-System Trading Partner generates
the application image, does the applicant also need to check the image
in Commons?
Although some System-to-System Trading Partner may have built their own system to generate applicant images, NIH strongly recommends that the applicant still check out the grant image of their assembled application in eRA Commons and not rely solely on the developer-generated image.
- Are there some optional fields in the schema that
are actually mandatory for the applicant on PureEdge?
Yes, the NIH team is discovering that there are many optional fields in the schema that the user is actually required to fill in on PureEdge. The NIH code is throwing system errors when we retrieve System-to-System submissions with empty tags in these fields. We will inform you as we learn of these so you are aware of them. Program Income on the 398 checklist and the Stem Cell tag are examples we have uncovered.
Communications
- Has there been outreach to the grantee community outside of the
NIH Guide for Grants and Contracts?
NIH staffers have reached out and will continue to reach out to the grantee community through websites, notices and presentations to professional associations such as the Society for Research Administrators (SRA), the National Council of University Research Administrators (NCURA), presentations and booths at scientific conferences, press releases, newsletter articles. Many staff are using signature blocks that give notice of the change, information is being added to e-mail notification of summary statement availability and prior approval letters, targeted emails will go out to business officials and principal investigators, "looking ahead" notice on funding opportunity announcements, etc. See http://era.nih.gov/ElectronicReceipt/communication.htm for more information.
- Where do we send applicants for information?
See http://era.nih.gov/ElectronicReceipt/support.htm.
- Has there been any outreach to the business community on electronic
submission?
There have been multiple notices in the NIH Guide for Grants and Contracts, we have posted information to our small business listserv (which includes some 11,000 names), and we will have a presence at many of the upcoming small business conferences. Please see http://era.nih.gov/ElectronicReceipt/communication.htm for more information.
Foreign Organizations
- I am an applicant who lives outside the U.S. and am unable to
access the Central Contract Registration (CCR) site. What should I do?
A few countries may have trouble accessing the CCR website. The applicant should send an email to security@bpn.gov and copy the NIH Electronic Submission mailbox at NIHElectronicSubmiss@mail.nih.gov.
- Are International Organizations required to hold a DUNS and register
in Grants.gov?
Yes.
- Can I register my organization in Commons in my native language
?
Unnfortunately, no; our policies require applicant organizations to:
- Register in English.
- use an English translation of their organization's name for the registration.
- Are there any tips to assist foreign organizations while registering
in eRA Commons?
Keep these handy pointers in mind while registering in eRA Commons. Applicant organizations:- Must have a DUNS number before registering in the eRA Commons. This DUNS number must match the DUNS number provided at CCR registration with Grants.gov.
- Must have a valid e-mail and should ensure that any filters on their email do not interfere with NIH email. Must also keep in mind that the sooner they reply to emails, the faster NIH can complete their registration.
- Some of the data fields in the 424 (R&R) do not really apply
to foreign organizations. How will this be handled?
For some of the data, special instructions are included in the Application Guide for foreign organizations. - Are International organizations required to obtain an EIN number as
part of the grant submission process?
NIH does not require international organizations to obtain an EIN number for
application submission. International organizations may use 44-4444444 for the
Employer Identification field in the SF424 (R&R) Cover Component of the
application package. [See NIH eSubmission Tips for International Applicants].
