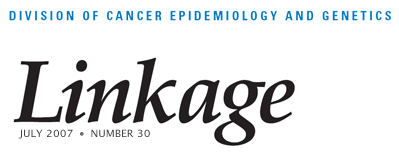Replication Strategies: The Key to Validating Genotype-phenotype Associations
As genome-wide association studies (GWAS) become more prevalent, identifying valid genotype-phenotype relationships among the multitude of positive results is an increasing challenge for epidemiology because so many findings are false positives. A common strategy to validate an initial finding is to use replication studies—cumulatively designed to confirm genotype-phenotype associations derived from candidate-gene analyses or GWAS.
Historically, research has focused mainly on discovering rather than validating genotype-phenotype associations. When replication studies are performed in independent datasets, most fail to reproduce initial findings, which might have resulted from chance alone, although poor study design, insufficient power, population stratification, and environmental or genetic heterogeneity could have played a role. With the transition from candidate-gene approaches to GWAS expected over the next few years, replication studies will become standard practice in confirming associations, eliminating false-positive findings, and narrowing the search for causal gene variants in different diseases.
To this end, NCI and the National Human Genome Research Institute (NHGRI) convened a working group to define a systematic approach to the design and dissemination of replication studies. Group members were drawn from several disciplines, including biostatistics, clinical medicine, epidemiology, genetics, and scientific publishing.
“This working group was born out of the urgent need for consistency,” said Stephen J. Chanock, M.D., Director of the Core Genotyping Facility at NCI. Dr. Chanock is the lead author of a feature article published in Nature in June titled “What constitutes replication of a genotype-phenotype association? Summary of an NCI NHGRI working group.”
The purpose of the article is to evaluate the role played by a single genetic association study; to propose criteria for establishing replication; and to define quality-control practices for statistical design and analysis, interpretation, and reporting of findings based on replication studies (see sidebar).
Suggested Criteria for a Positive Replication Study
- Sufficient sample size
- Well-powered, independent data sets
- Trait studied is similar/identical to one in the initial study
- Strong rationale for the choice of trait studied
- Significance of outcome should be similar to that in the initial study
- Smaller p value should be determined, if possible
- Genetic model should be consistent between studies
- Detailed report of study design and data analysis
Dr. Chanock and his colleagues recently used a staged replication strategy to validate a genetic link between a region in chromosome 8q24 and prostate cancer risk. This study, along with two other reports of similar results, appeared online in Nature Genetics on April 1. The NCI team began with a nested case-control study of more than 550,000 single nucleotide polymorphisms (SNPs) in more than 1,100 individuals with prostate cancer and an equal number of matched controls. The study is part of the Cancer Genetic Markers of Susceptibility (CGEMS) project briefly described in a review of GWAS by Hunter and colleagues in Cancer Causes and Control (2007;18:479–484). The initial analysis was followed by four additional studies totaling more than 3,000 patients and 3,000 matched controls.
Dr. Chanock noted, “The advantage of CGEMS is that it involves large-scale efforts of consortia involving intramural and extramural scientists joining forces to pool data from multiple population cohorts and case-control studies. This collaborative effort meets the need for rapid replication of findings in different population groups, with each subsequent stage refining the number of genetic loci that are likely to contribute to cancer risk.” The final data for all CGEMS studies are made publicly available through NCI’s cancer Biomedical Informatics Grid (caBIG™).
The combined analysis of prostate cancer not only confirmed two previous reports—one from deCODE Genetics in Iceland and another from a team at the University of Southern California and the Broad Institute at Harvard University and the Massachusetts Institute of Technology—of a SNP (rs1447295) on chromosome 8q24, but it also identified a second independent variant (rs6983267) in the same region that conveyed a higher population attributable risk (21%) than the earlier marker (9%) among men of European ancestry.
Curiously, the SNPs identified in the prostate cancer scans are located in a region of 8q24 that has few known or predicted genes. Further work is obviously needed to narrow the search for causal variants through fine-scale mapping, resequencing, and functional studies. Nevertheless, the findings to date illustrate the power of large-scale initiatives of consortia with replication studies guided by the criteria formulated by the NCI-NHGRI working group.
While the working group report outlines appropriate strategies to discern legitimate genotype-phenotype associations, it also addresses the importance of rapidly disseminating high-quality information to the scientific community. In addition, the report encourages journal editors to publish replication studies that either validate or refute genotype-phenotype associations. Dr. Chanock noted how difficult it is to “wade through the sea of information on single candidate genes” and “the need to develop reporting mechanisms to ensure that associations later found to be false positives are not re-explored by other investigators, which wastes time and resources.”
There are currently several GWAS involving prostate, breast, and pancreatic cancers that are using resources from the NCI Cohort Consortium and some case-control studies. Also in the planning stages at NCI are GWAS for bladder, lung, and kidney cancers as well as lymphoma. However, there remains a large gap between the findings from whole-genome scans and the detection of causal genetic variants and mechanisms, which should eventually inform clinical studies aimed at risk prediction, early detection, and preventive or therapeutic interventions as well as epidemiologic studies into gene-gene and gene-environment interactions aimed at a fuller understanding of cancer causation. This sequence of follow-up studies will be triggered not by a single association study but rather by a well-designed replication strategy involving multiple coordinated studies.
According to Dr. Chanock, “Many people have declared victory way too early, when validation should be the next step to conclusively relate genotype to phenotype.”
—Amber K. Boehm, Ph.D.

Maria Teresa Landi and Ruth Pfeiffer
InvesTIGaTors reCenTly awarDeD Tenure
NIH recently awarded scientific tenure to Maria Teresa Landi, M.D., Ph.D., of the Genetic Epidemiology Branch (GEB) and Ruth M. Pfeiffer, Ph.D., of the Biostatistics Branch (BB).
After training in medical oncology, occupational medicine, and epidemiology, Dr. Landi became a tenure-track investigator in GEB in 1999. Her research has focused on the role of genetic susceptibility factors in tumors with known environmental determinants, such as melanoma and lung cancer, using association and linkage studies. She has also investigated the effects of dioxin on the risk of cancer and other outcomes for the population exposed in an industrial accident in Seveso, Italy.
After receiving a doctorate in mathematical statistics, Dr. Pfeiffer joined BB in 1999 as a
postdoctoral fellow. She became a tenure-track investigator in 2001 and has conducted research
on multilevel random effects models, epidemiologic study design, and mixture or latent class
models. Much of her methodological research grew out of collaborations with scientists throughout
DCEG, and many of the tools Dr. Pfeiffer developed have been applied subsequently by
researchers in DCEG and elsewhere.
—B.J. Stone, Ph.D.
vIsITInG sCholars ProGram hosTs Three PromInenT sCIenTIsTs
To kick off the 2007 Visiting Scholars Program, DCEG proudly hosted three leading epidemiologists. Initiated in 2004, the program promotes intramural-extramural scientific collaboration and idea-sharing through intensive two-day visits that include a keynote seminar, scientific roundtables and discussions, one-on-one meetings, and sessions with DCEG fellows and women scientists. The DCEG Visiting Scholar Award recognizes leadership and vision in epidemiology and public health.
In January, DCEG welcomed Dr. Elizabeth A. Holly, Professor and Head of the Cancer Epidemiology Division at the University of California, San Francisco (UCSF). After receiving a Ph.D. in epidemiology from the University of California, Berkeley, Dr. Holly was a postdoctoral fellow at the University of Washington, studying reproductive and hormonal risk factors for melanoma under the mentorship of Dr. Noel Weiss. Later as an epidemiologist at the Northern California Cancer Center, she expanded her research portfolio to include projects on virally related cancers, particularly those associated with HPV and HIV infection. In 1991, Dr. Holly joined the faculty at UCSF and launched a number of large, ambitious case-control studies on HIV natural history, melanoma, childhood brain tumors, and pancreatic cancer, underscoring her diverse research interests and expertise.

Joseph Fraumeni, Elizabeth Holly, and Margaret Tucker.
In her Visiting Scholar seminar, titled “Risk factors for pancreatic cancer in a population-based case-control study in the San Francisco Bay Area,” Dr. Holly described the project, launched in 1994, as the largest case-control study of pancreatic cancer at the time, involving 532 pancreatic cancer cases and 1,701 population-based controls. A rich resource for providing clues about this poorly understood cancer, the study’s major strengths lay in the design and detailed questionnaire that ascertained data on several suspected genetic, immunologic, and dietary factors previously thought to have etiologic relevance for pancreatic cancer. Importantly, because no proxy interviews were conducted, the bias typically encountered with indirect data collection methods was minimized. Dr. Holly highlighted a detailed analysis revealing a dose-dependent, protective effect of allergies on pancreatic cancer risk, suggesting a role for immunologic factors that merits further investigation.
During the remainder of her visit, which was hosted by Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of the Genetic Epidemiology Branch (GEB), Dr. Holly attended a series of roundtable discussions related to the Division’s work on lung cancer, pancreatic cancer, melanoma, lymphoma, and HPV-related cancers. These sessions were moderated, respectively, by DCEG scientists Neil E. Caporaso, M.D. (GEB), Rachael Stolzenberg-Solomon, M.P.H., Ph.D., Nutritional Epidemiology Branch (NEB), Alisa M. Goldstein, Ph.D. (GEB), Ola Landgren, M.D., Ph.D. (GEB), and Philip E. Castle, Ph.D., M.P.H., Hormonal and Reproductive Epidemiology Branch (HREB). Dr. Holly also participated in a meeting of DCEG Women Scientists, where principal investigators, staff scientists/ clinicians, and fellows discussed topics related to mentoring and career advancement. Dr. Holly commented on the benefits of having a large group of cancer epidemiologists with various areas of expertise conveniently located in one institution. “It’s a wonderful opportunity to discuss scientific and professional ideas,” she stated, stressing that “postdoctoral fellows should be encouraged, even expected, to ask questions and work with investigators in different areas to gain experience and new skills.”
It is noteworthy that, among her honors and achievements, Dr. Holly is recognized as being among the top 5 percent of NIH grant awardees since 1981.
Dr. Walter C. Willett, Fredrick John Stare Professor of Epidemiology and Nutrition and chair of the Department of Nutrition at the Harvard School of Public Health, was hosted in February by Demetrius Albanes, M.D., a senior investigator in NEB and Chief of the Office of Education. A graduate of the University of Michigan Medical School and Harvard School of Public Health, Dr. Willett has been a pivotal leader in the field of nutritional epidemiology and in designing large prospective cohort studies for the evaluation of chronic diseases of public health importance.

Walter Willett
After receiving a doctorate in epidemiology at Harvard, Dr. Willett remained on the faculty and, in 1980, became co-investigator for the Nurses’ Health Study (NHS), a cohort of 122,000 nurses between ages 30 and 55 years, established in 1976 by Dr. Frank Speizer to determine the long-term health effects of oral contraceptive use in women. Dr. Willett introduced a food-frequency questionnaire (FFQ) module into the regular two-year follow-up NHS questionnaires, and by 1986, the FFQ was incorporated into the follow-up protocol every four years. The cohort data have been used in hundreds of analyses and combined in major pooled studies, including the Harvard Dietary Pooling Project and NCI Breast and Prostate Cancer Cohort Consortium (BPC3).
Dr. Willett’s honors include election into the Institute of Medicine of the National Academies, the General Motors Cancer Research Foundation Charles S. Mott Prize, and the American Cancer Society Medal of Honor. He has served on the editorial boards of several major peer-reviewed journals, such as the New England Journal of Medicine, Cancer Research, and the American Journal of Epidemiology.
Historically, efforts in nutritional epidemiology have experienced significant challenges resulting from limitations of dietary assessment tools, recall bias, and the co-linearity of many potential confounders, the sum of which often results in the detection of modest, attenuated, or inconclusive associations.
In his Visiting Scholar seminar, titled “Diet and breast cancer: The search for veritas,” Dr. Willett described the decades-long work using data from the NHS, NHS-II, and Health Professionals’ Follow-up Study to clarify the relationship between diet and cancer. Historically, efforts in nutritional epidemiology have experienced significant challenges resulting from limitations of dietary assessment tools, recall bias, and the co-linearity of many potential confounders, the sum of which often results in the detection of modest, attenuated, or inconclusive associations. Using the association between dietary fat and breast cancer as an example, Dr. Willett described the complexity of these studies and the difficulty in establishing a clear relationship utilizing a large cohort study design. Studies looking at percentage of energy from fat and breast cancer incidence have shown no association, and analyses of fat subtypes have also been inconclusive. Dr. Willett pointed out that large clinical trials designed to reduce breast cancer incidence by lowering fat intake have not shown significant effects. He concluded by stating, “No single study will provide the complete picture. The best information about the association between diet, lifestyle, and cancer risk and prevention will come from the combined results of long-term prospective studies and controlled trials of intermediate endpoints.”
During the remainder of his visit, Dr. Willett met with investigators from DCEG and participated in roundtable discussions. In a session moderated by Arthur Schatzkin, M.D., Dr.P.H., Chief of NEB, Dr. Willett shared his experiences in assessing dietary intake in epidemiologic investigations, using intensive sub-studies in evaluating and adjusting for dietary measurement error, and refining existing instruments. HREB fellow Mia M. Gaudet, Ph.D., led a session on the interactive effects of hormones and obesity on the risk of endometrial and breast cancers. Michael F. Leitzmann, M.D., Dr.P.H., a tenure-track investigator in NEB, facilitated discussions about DCEG studies on body size in relation to cancer and total mortality. Dr. Willett wrapped up his visit with an informal session with fellows, sharing his ideas about future areas of interest in cancer epidemiology, including gene-environment interaction, characterization of tumors more fully, identification of factors affecting progression of tumors after diagnosis, understanding effects of exposure across the lifespan, and global cancer health. In the era of big science, Dr. Willett encouraged fellows to become involved in efforts of consortia and be creative when exploring projects. “You should be opportunistic and find new ways to use existing resources; everybody wins in that situation.”
Dr. Brian E. Henderson, Dean of the University of Southern California (USC) Keck School of Medicine and Kenneth T. Norris, Jr., Cancer Prevention Chair, visited DCEG in March. A graduate of the University of California, Berkeley, and the University of Chicago Medical School, Dr. Henderson began his career as a medical officer in the U.S. Public Health Service and later led the CDC Arbovirology Unit and the WHO Regional International Center for Arboviruses in the Americas. In 1970, Dr. Henderson became an associate professor in the Department of Pathology at USC and began working in cancer epidemiology. After just eight years, he became founding chair of the Department of Preventive Medicine and later went on to direct the Norris Comprehensive Cancer Center in Los Angeles.
Known for his innovative epidemiologic and interdisciplinary approaches, Dr. Henderson was on the forefront of several breakthroughs in epidemiologic research, including the role of menopausal hormones and susceptibility genes in cancer etiology. He was also one of the first to promote the development of “productive resources” through his creation of cancer registries in Los Angeles and countries of the Pacific Rim, which allowed for rapid case identification for epidemiologic research.

Brian Henderson
In his Visiting Scholar seminar, titled “The genetics of prostate and breast cancer,” Dr. Henderson described the candidate gene and admixture mapping approaches employed in the Multiethnic Cohort Study (MEC). Begun in 1998, the study enrolled more than 215,000 participants between the ages of 45 and 75 years from Hawaii and Los Angeles to investigate dietary and other lifestyle determinants of cancer and to explore how these associations vary across ethnic groups. African American, Latino, Native Hawaiian, and white participants received a 26-page questionnaire upon enrollment and two subsequent follow-up surveys, providing data on nutrition, physical activity, smoking, and alcohol use. Biospecimens, including DNA, were also collected, and cancer outcomes and deaths were ascertained through record linkage to cancer registries. To date, numerous analyses have been published on findings from this study, most recently the discovery of prostate cancer susceptibility loci in the 8q24 region of the genome. MEC data have also been a valuable resource for BPC3 to conduct replication studies and clarify the role of genetic susceptibility in the development of breast and prostate cancers.
Dr. Henderson encouraged junior researchers to become involved in large consortia and genome-wide association studies. “There’s more than enough work to do,” he stated, adding that fellows have much to offer in these collaborative projects that often produce more opportunities than investigators have time to explore.
After the seminar, Dr. Henderson participated in several roundtable discussions, the first of which focused on “Multiethnic approaches to genetic and environmental causes of cancer,” hosted by Robert N. Hoover, M.D., Sc.D., Director of the Epidemiology and Biostatistics Program. Richard B. Hayes, D.D.S., Ph.D., a senior investigator in the Occupational and Environmental Epidemiology Branch, moderated a series of presentations on the causes of prostate cancer, followed by a session on breast cancer led by Louise A. Brinton, Ph.D., Chief of HREB. In a lunch session with DCEG fellows, Dr. Henderson encouraged junior researchers to become involved in large consortia and genome-wide association studies. “There’s more than enough work to do,” he stated, adding that fellows have much to offer in these collaborative projects that often produce more opportunities than investigators have time to explore. He also encouraged fellows to receive training in molecular biology to complement their epidemiology backgrounds and to improve their understanding of the nomenclature and mechanisms involved in molecular and genetic epidemiologic studies.
—Alyssa Minutillo, M.P.H.
new Fellows’ ColloquIum FosTers CollaboraTIon anD neTworkInG
D
CEG is an ideal setting for postdoctoral research. With abundant scientific resources and excellent mentors, the Division has more than 70 fellows from diverse educational and research backgrounds. At the 2006 Fellows’ Town Hall meeting, fellows expressed a desire for more opportunities to interact intellectually, professionally, and socially with other fellows across the Division. As a result, a monthly DCEG Fellows’ Colloquium was instituted.
Organized by a planning committee of fellows from each branch and supported by DCEG’s Office of Education, the colloquium series has gotten off to an excellent start. At the January meeting, Division Director Joseph F. Fraumeni, Jr., M.D., joined the fellows for a pizza lunch and expressed his enthusiasm for the colloquium and its role in promoting interactions, identifying research opportunities, and facilitating scientific collaborations. The fellows discussed ideas for the general format and subject matter of subsequent sessions. February’s colloquium focused on promoting research independence and featured fellows who had recently won DCEG Intramural Research Awards. In March, two senior investigators and one postdoctoral fellow drew on their personal experiences to discuss how to start an international study.

DCEG fellows (Photograph Credit: Ernie Branson)
Future colloquia will be held on the last Friday of each month and led by each branch on a rotating schedule. The sessions will focus on a broad range of topics, including fellows’ research, methodology, and skill-specific tutorials. A fellows’ scientific retreat is being planned to further facilitate intellectual and social interaction.
To ensure that the colloquium series meets the needs of the fellows, comments and suggestions should be sent to representatives Elizabeth C. Bluhm, M.D., M.P.H. (Radiation Epidemiology Branch), Neal D. Freedman, Ph.D., M.P.H. (Nutritional Epidemiology Branch), Mia M. Gaudet, Ph.D. (Hormonal and Reproductive Epidemiology Branch), Dean Hosgood, M.P.H. (Occupational and Environmental Epidemiology Branch), Jill Koshiol, Ph.D. (Genetic Epidemiology Branch), Rayna Matsuno, M.S. (Biostatistics Branch), Christine M. Mueller, D.O. (Clinical Genetics Branch), or Hui-Lee Wong, Ph.D. (Viral Epidemiology Branch).
—Neal D. Freedman, Ph.D., M.P.H., Mia M. Gaudet, Ph.D., and Jill Koshiol, Ph.D.
workshoP PromoTes researCh on aIDs-assoCIaTeD CanCers
In January, DCEG, the NCI Office of AIDS Malignancy Program (OAMP), and Tata Memorial Center (TMC) of Mumbai, India, cosponsored a five-day workshop titled, “Research methodologies for study of AIDS-related malignancies,” held in Mumbai.Dr. Kishor Bhatia, Director of OAMP, and Dr. Geraldina Dominguez, Program Director of OAMP, coordinated the NCI efforts. Sam M. Mbulaiteye, M.D., a tenure-track investigator in the Viral Epidemiology Branch (VEB), and Dr. Aruna Alahari Dhir, head of the Department of Medicine at TMC, cochaired a session on “AIDS-cancer match registry studies.” Other DCEG participants included Robert J. Biggar, M.D., a senior investigator in VEB, Anil K. Chaturvedi, Ph.D., a postdoctoral fellow in VEB, and Hormuzd A. Katki, Ph.D., a staff scientist in the Biostatistics Branch.
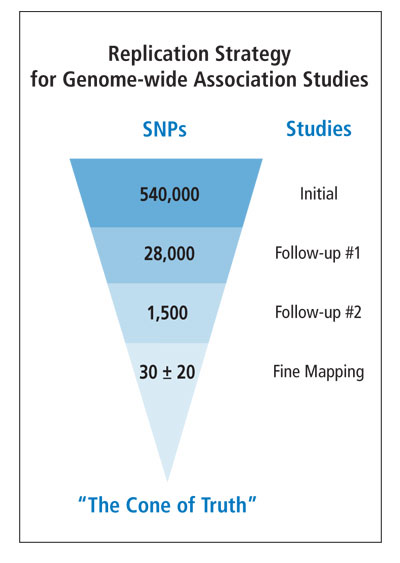
The idea for the workshop came from discussions at an NCI AIDS Malignancies Working Group meeting in Bethesda in 2006. The working group was charged with assessing the feasibility and enthusiasm for collaborative studies on HIV/AIDS and cancer in India. Attendees came from all parts of India for state-of-the-art lectures and technology transfer workshops from internationally recognized experts. Plenary sessions featured both American and Indian researchers, who provided their unique perspectives on the AIDS-cancer epidemic in their respective countries. Dr. Biggar gave a plenary lecture on “HIV/AIDS, immunity, and cancer,” and Dr. Bhatia discussed the challenges and opportunities for developing collaborative studies to address the impact of HIV/AIDS on cancer in India.
Breakout sessions covered such core topics as epidemiology, laboratory studies of oncogenic viruses, pathology, and immunity. The epidemiology workshop attracted 23 participants, including pathologists, clinicians, and cancer registry personnel from 10 of India’s 28 states. Dr. Nanda Kumar (India National Cancer Registry Program), Dr. Ajay Khera (National AIDS Control Program), Dr. Dhir, and Dr. Umar Tendolkar (Mumbai AIDS Society) shared their experiences, noting the challenges of conducting cancer research in HIV-infected populations in India.
DCEG scientists led the “AIDS-cancer match registry studies” session, discussing research opportunities, record-linkage methods, and ethics. Dr. Biggar led the ethics discussion. Dr. Chaturvedi and Dr. Rajesh Dixit (TMC) discussed methodological issues for statistical analysis in registry-linkage studies, and Drs. Mbulaiteye and Katki led discussions on methods used to study HIV-associated cancers in international settings.

NCI Workshop Co-organizers: Hormuzd Katki, Anil Chaturvedi, Kishor Bhatia, Robert Biggar, Sam Mbulaiteye,
and Geraldina Dominguez.
DCEG investigators also visited and reviewed research opportunities at three hospitals in Hyderabad, Andhra Pradesh State. Of special interest was the L.V. Prasad Eye Institute, which treats a large number of patients with ocular lymphoma and squamous cell carcinoma of the conjunctiva, a rare cancer in the United States, but associated with AIDS in tropical Africa. DCEG investigators discussed with local researchers and clinicians the unique research infrastructure and opportunities for collaborative studies, including a pilot study to determine the feasibility of linking HIV/AIDS and cancer registries and to characterize the association between HIV infection and ocular cancers.
—Sam M. Mbulaiteye, M.D.

Chordoma workshop participants (Photograph Credit: Maggie Bartlett)
workshoP exPlores ChorDoma researCh
In May, several members of DCEG’s Genetic Epidemiology Branch participated in the First International Chordoma Research Workshop in Bethesda, hosted by the Chordoma Foundation in collaboration with NCI, the National Human Genome Research Institute, the National Institute of Neurological Disorders and Stroke, and the NIH Office of Rare Diseases. The purpose of the workshop was to develop an action plan for advancing chordoma research and to establish the necessary collaborations.
Mary Lou McMaster, M.D., gave an overview titled “The epidemiology of chordoma,” and Rose Yang, Ph.D. M.P.H., presented results on a “Linkage analysis to map major susceptibility genes for familial chordoma.” Dilys M. Parry, Ph.D., a member of the organizing committee, co-moderated a breakout group session titled “Mechanisms of disease.”
The workshop included a multidisciplinary group of international experts actively involved in chordoma research, along with scientists who have made breakthroughs or established new paradigms for studying rare tumors in their respective fields. The Second International Chordoma Research Workshop is scheduled for April 2008.
DunsTana melo sTuDIes IoDIne anD CesIum exPosures

Dunstana Melo
In 2006, Dunstana Rabelo de Melo Ph.D., joined the Radiation Epidemiology Branch as an Oak Ridge Institute for Science Education Senior Fellow. Dr. Melo specializes in assessment of exposure doses to ionizing radiation. She is a consultant for the United Nations Scientific Committee on Effects of Atomic Radiation on occupational exposure to ionizing radiation and for the International Atomic Energy Agency on developing standards of protection for pregnant workers.
At DCEG, Dr. Melo is working on a dose assessment project among patients treated with iodine-131. “We have iodine measurement data for about 3,000 patients, and I will use them to develop a biokinetic model that describes the behavior of iodine in the body,” she said. “The idea is to develop a generic model taking into account the modifying factors, so the model can be applied to patients with hyperthyroidism or hypothyroidism and to healthy people.”
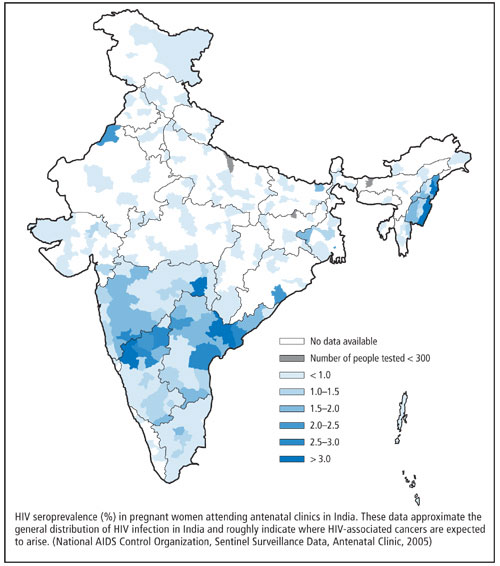
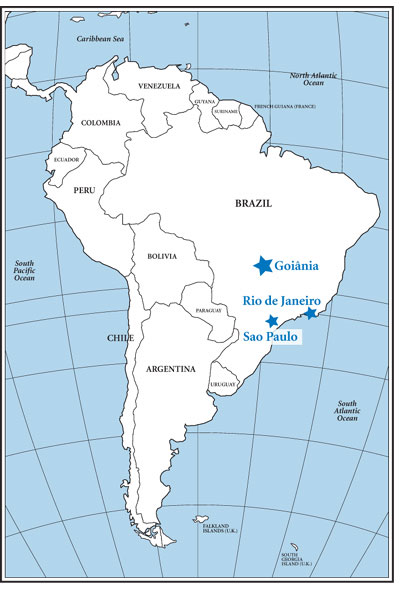 Goiânia is located on the central Brazilian plateau,
838 miles from Rio de Janeiro and 571 miles from
Sao Paulo, where the major radiological protection
resources are located.
Goiânia is located on the central Brazilian plateau,
838 miles from Rio de Janeiro and 571 miles from
Sao Paulo, where the major radiological protection
resources are located.
The model would be useful after accidents involving iodine-131 (such as Chornobyl) and relevant to possible acts of radiological terrorism. Iodine-131, one of the most common radionuclides, is already fairly widespread due to nuclear tests and fallout.
Dr. Melo was part of the team that evaluated the exposure of people involved in the 1987 cesium-137 incident in Goiânia, Brazil. Two men broke into an unused hospital and removed parts of a radiation therapy unit, thinking they could sell the parts. A canister of cesium-137 was broken, releasing the radioactive powder, which has a half-life in the environment of 30 years. The powder was disseminated around the city by the men and their families, friends, and acquaintances. The resulting decontamination effort covered 40 city blocks and cost upward of $20 million. More than 112,000 people were monitored.
At the time, Dr. Melo was working at the Brazilian Nuclear Energy Commission’s Institute of Radiation Protection and Dosimetry, where she worked for 22 years. “We were responsible for monitoring workers who handle sources of radiation,” she said. “I was responsible for the dose assessment of those workers. We also had some projects evaluating the dose of the population living in areas of high background radiation.”
Dr. Melo and her colleagues calculated internal doses for each contaminated person from the Goiânia incident. “About 130 people were contaminated, and some had very high levels of internal contamination. Four of them died. The accident is now considered one of the most serious of its kind in the Western Hemisphere. It led to a complete revision of Brazilian regulations relevant to using and storing radiation sources.”
Patients were treated with Prussian blue to enhance cesium elimination from their bodies. “We also evaluated the efficacy of that treatment,” she said. Prussian blue reduces the biological half-life of cesium in the body from about 110 days to about 30 days. It was first produced in the 1700s as a blue pigment and was used to dye Prussian military uniforms.
Though relatively new to the United States—born and raised in Brazil, Dr. Melo previously spent six months in the United States while working on her doctorate at the Inhalation Toxicology Research Institute in New Mexico—she is enjoying DCEG. “I like the work I’m doing, and I’m enjoying the interactions with people,” she said. “They’re open and friendly. It’s been a very good experience.”
Dr. Melo has a B.S. in biology and an M.S. and Ph.D. in biophysics from the Federal University of Rio de Janeiro. She has authored more than 60 publications and spoken at more than 30 conferences and meetings.
—Nancy Volkers
reTreaT PromoTes work oF sTaFF sCIenTIsTs anD sTaFF ClInICIans
On February 12, 2007, DCEG held its third staff scientist/staff clinician (SS/SC) retreat, sponsored by the Committee of Scientists (COS) and organized by COS representatives Mark J. Roth, M.D., Nutritional Epidemiology Branch, and Catherine Schairer, Ph.D., Biostatistics Branch. Twenty-eight SS/SCs attended the half-day retreat, along with Joseph F. Fraumeni, Jr., M.D., Division Director, Shelia Hoar Zahm, Sc.D., Deputy Division Director, Robert N. Hoover, M.D., Sc.D., Director of the Epidemiology and Biostatistics Program, Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of the Genetic Epidemiology Branch, Donna Siegle, Manager of the DCEG Administrative Resource Center, and Aaron E. Blair, Ph.D., M.P.H., COS chair and a senior investigator in the Occupational and Environmental Epidemiology Branch.
The retreat’s primary objectives were to enhance understanding of the important roles SS/SCs play in the Division’s multifaceted research program and to discuss ways that they can enhance their intellectual, scientific, and career growth. Dr. Fraumeni opened the retreat with a discussion on the importance of value-added science, the general role of SS/SCs at NIH, and their unique role in DCEG. He described the Division’s initiatives to recognize SS/SCs, including the annual Outstanding Research Paper by an SS/SC Award, representation on COS, eligibility as alternate Women Scientist Advisor (a new initiative), eligibility for the loan repayment program, participation in the NCI Intramural Scientific Retreat, and dedicated town meetings and retreats with SS/SCs.
Dr. Hoover added that most SS/SCs have a major leadership role in specific aspects of the Division’s studies and emphasized the importance of SS/SCs striving to advance their scientific fields, such as exposure assessment, potential uses of the Internet, biologic sample collection, implementation of innovative clinical protocols, and the development of information technology and analytical tools. He concluded, “Your growth is vital to the Division’s growth.”

Staff scientist/staff clinician retreat participants
Dr. Zahm reviewed the three appointment mechanisms for SS/SCs: Title 42, now the predominant mechanism; general schedule (GS) appointments, which are rare unless an individual is already in this category; and PHS Commission Corps appointments. She also discussed the pay models and opportunities for promotion in each mechanism.
Dr. Tucker then reviewed the Division’s philosophy and commitment to career development in the context of programmatic research efforts. She highlighted a number of training opportunities for SS/SCs, including the Graduate Partnership Program, which may be available to support a doctoral degree, and online courses through HHS University. Branches may also cover medical board recertification courses for SCs.
Finally, Ms. Siegle reviewed the implementation of the new Performance Management Appraisal Plan, particularly as it relates to SS/SCs. The purpose of the new plan is to link pay to performance more effectively. Dr. Zahm explained that “exceptional” ratings will be rare and will reflect special accomplishments or events during the review year. Since many questions and concerns were raised at the retreat about this new system, Ms. Siegle offered to form a working group to develop an appropriate planning process and evaluation criteria for SS/SCs. The group has already met on several occasions, and the development of revised criteria is well under way and will be shared with the SS/SC community for comments.
The retreat concluded with a discussion among SS/SCs about issues of mentoring, authorship, recognition, and the need for DCEG representation on the NIH-wide SS/SC committee. Recommendations based on the retreat will be made by COS to the DCEG Office of the Director.
—Mark J. Roth, M.D., and Catherine Schairer, Ph.D.
DCeG ParTICIPaTes In The CenTennIal aaCr meeTInG
In April, many DCEG researchers participated in the centennial American Association for Cancer Research (AACR) meeting at the Los Angeles Convention Center. The series of events included award lectures, exhibits, plenary sessions and symposia, and poster sessions. During an opening address, John E. Niederhuber, M.D., NCI Director, focused on NCI’s current fiscal realities and outlined the current strategy for continuing its vital scientific momentum. He updated AACR members on the Institute’s budget challenges and scientific advances and opportunities in cancer research.
Approximately 30 posters involving DCEG staff were selected for presentation. Six of the Division’s researchers were recognized with an award or by the press. Anil K. Chaturvedi, Ph.D., a postdoctoral fellow in the Viral Epidemiology Branch (VEB), was a recipient of the AACR-Bristol-Myers Squibb Scholar-in-Training Award and presented his research on “Second cancers among 104,760 survivors of cervical cancer: Evaluation of long-term risk” at a press conference. Kim N. Danforth, Sc.D., a Sallie Rosen Kaplan Fellow in the Hormonal and Reproductive Epidemiology Branch (HREB), Mark Purdue, Ph.D., a research fellow in the Occupational and Environmental Epidemiology Branch (OEEB), and Hui-Lee Wong, Ph.D., an Oak Ridge Research Associated Universities Fellow in VEB, won AACR-Merck Scholar-in-Training Awards and presented their work on “Variants in inflammation-related genes and risk of prostate cancer: The PLCO study,” “Genetic variation in the inhibin pathway and risk of testicular germ cell tumors,” and “Cytokine signaling pathways and polymorphisms and risk of AIDS-related lymphoma” and “Insulin-like growth factor promoter polymorphisms and colorectal cancer: A functional genomics approach,” respectively.
Neal D. Freedman, Ph.D., M.P.H., a postdoctoral fellow in the Nutritional Epidemiology Branch (NEB), presented his findings on “Fruit and vegetable intake and head and neck cancer in a large United States prospective cohort study” at a press conference. Deukwoo Kwon, Ph.D., a postdoctoral fellow in the Radiation Epidemiology Branch (REB), was selected for the AACR-National Ovarian Cancer Coalition Scholar-in-Training Award and presented his poster on “Identifying biomarkers from mass spectrometry data with ordinal outcomes.” Jocelyn M. Weiss, Ph.D., M.P.H., a postdoctoral fellow in OEEB, received the AACR-AstraZeneca Scholar-in-Training Award and presented her work on “Hormonal risk factors for lung cancer in female lifetime nonsmokers from Shanghai, China.”
Other DCEG Presenters
|
|
workshoP InvesTIGaTes vITamIn D anD CanCer rIsk
In May, DCEG, the Division of Cancer Prevention (DCP), the Division of Cancer Control and Population Studies (DCCPS), and the Office of Dietary Supplements (ODS) cosponsored a two-day international workshop on “Vitamin D and cancer: Current dilemmas/future needs.” Organizers of the workshop were Dr. Cindy D. Davis and Dr. John Milner (DCP); Dr. Virginia Hartmuller (DCCPS); Patricia Hartge, Sc.D., Deputy Director of the DCEG Epidemiology and Biostatistics Program and Special Assistant to the DCCPS Director; D. Michal Freedman, Ph.D., M.P.H., Radiation Epidemiology Branch; and Dr. Mary Frances Picciano and Dr. Christine Swanson (ODS). About 180 scientists attended the sessions.

Workshop Co-organizers: (front) D. Michal Freedman, John Milner, and Cindy Davis; (back) Virginia Hartmuller, Patricia Hartge, and Mary Frances Picciano.
The goals of the workshop were to evaluate the scientific evidence related to vitamin D and cancer risk, specify gaps in knowledge, and identify research needed to make recommendations for cancer prevention.
The first session, moderated by Arthur Schatzkin, M.D., Dr.P.H., Chief of the Nutritional Epidemiology Branch, focused on the epidemiologic evidence linking vitamin D with cancer risk. Speakers included Dr. Gary G. Schwartz from Wake Forest University School of Medicine, who provided an overview of critical factors to evaluate dietary vitamin D and sunlight, the principal source of vitamin D, and cancer risk; Dr. Edward Giovannucci from the Harvard School of Public Health, who addressed epidemiologic studies of colorectal and prostate cancer risk; Dr. Thomas E. Rohan from the Albert Einstein Cancer Center, who discussed the weight of the evidence on breast cancer risk; and Margaret A. Tucker, M.D., Director of the Human Genetics Program and Chief of the Genetic Epidemiology Branch, who addressed the difficulties in assessing sun exposure and potential public health dilemmas in light of the known carcinogenicity of such exposure. Later sessions covered the role of nutrigenetics and other dietary components in modifying the potential relationship between vitamin D and cancer risk.
The organizers will publish the proceedings of the workshop, and plans are under way in DCEG to use the Division’s cohorts to explore the protective effect of vitamin D on sunlight exposure and cancer risk.
—D. Michal Freedman, Ph.D., M.P.H.
MOBILE RISK ASSESMENT TOOLS NOW AVAILABLE
Many studies in the DCEG research portfolio focus on assessing cancer risk and developing algorithms to estimate this risk. Two such tools developed by the Division and designed for use by health professionals include the Gail model, also known as the Breast Cancer Risk Assessment Tool (BCRA), and the Melanoma Risk Assessment Tool (MRAT). This summer, DCEG announced the availability of downloadable files for installation of these tools onto personal digital assistant devices, which are becoming increasingly common in the practice of general medicine. The mobile versions were introduced at the 2007 meeting of the American Society of Clinical Oncology in Chicago. Over the coming months, the Division will distribute information about the mobile tools to health care providers across the country through targeted mailings and attendance at national meetings. The tools and their mobile versions are available on the NCI web site:
- BCRA is available at www.cancer.gov/bcrisktool.
- MRAT is available at www.cancer.gov/melanomarisktool.
To download the tools to your handheld device, click on Mobile Access.
—Jennifer Loukissas, M.P.P.
DCeG sTaFF reCoGnIzeD aT Town meeTInG
John E. Niederhuber, M.D., NCI Director, was the featured speaker at the 2007 DCEG annual town meeting held in April. After an introduction by Joseph F. Fraumeni, Jr., M.D., DCEG Director, Dr. Niederhuber recognized the Division’s unique role at NCI, with epidemiology providing the first step in cancer prevention by developing the scientific basis for understanding its causes. He emphasized his commitment to DCEG’s efforts in genomics and expressed enthusiasm for future projects. Furthermore, Dr. Niederhuber commended the Division for its extramural collaborations, which have developed valuable epidemiologic and informatics resources. Dr. Niederhuber also shared his research priorities for the upcoming year, which include integrated profiling methods, such as the Cancer Genetic Markers of Susceptibility (CGEMS) study.
Following Dr. Niederhuber’s remarks, Shelia Hoar Zahm, Sc.D., DCEG Deputy Director, led the annual awards ceremony that recognizes outstanding service and scientific contributions over the past year. The Division was commended for its generosity to the Combined Federal Campaign (CFC), which benefits nonprofit charities in the local area and around the world. In 2006, DCEG received its ninth consecutive CFC Presidential Award for meeting 129 percent of its dollar goal, contributing approximately $32,300, and 80 percent of its participation goal. Elyse Wiszneauckas, Office of Division Operations and Analysis (ODOA), was recognized for her leadership in coordinating the Division’s contributions to the 2006 CFC, as were Branch key workers: Cherise Banks and Tawanda Roy, Nutritional Epidemiology Branch (NEB), Sonja I. Berndt, Pharm.D., Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), Holly Brown, Biostatistics Branch, Patricia Chandler (ODOA), Jennifer Connor, Hormonal and Reproductive Epidemiology Branch (HREB), Sadie Holmes-Lillie, Genetic Epidemiology Branch (GEB), Jenna Nober, Radiation Epidemiology Branch (REB), José Reyes, Clinical Genetics Branch (CGB), Julie Grey, Viral Epidemiology Branch, and Rashida Williams, DCEG Administrative Resource Center.
DCEG Intramural Research Awards (IRAs), competitive research funding awards of up to $50,000, were given to Parveen Bhatti, Ph.D. (REB), for his proposal titled “Assessing DNA damage and telomere length in biologic samples collected before and after cancer diagnoses”; Dr. Berndt, for her project on “Genetic variation in transcription factor genes and prostate cancer risk”; Jonine D. Figueroa, Ph.D., M.P.H. (HREB), for her proposal titled “Evaluation of estrogen receptor coregulators with breast cancer risk and tumor characteristics”; Neal D. Freedman, Ph.D., M.P.H. (NEB), for his proposal on “Alcohol metabolites and esophageal cancer risk”; Jill Koshiol, Ph.D., M.P.H. (GEB), for her project on “Evaluation of the presence and functionality of human papillomavirus in esophageal squamous cell carcinoma”; Unhee Lim, Ph.D. (NEB), for her proposal titled “A nested case-control study of genomic DNA methylation and the risks of non-Hodgkin lymphoma and multiple myeloma in the PLCO trial”; and Sharon A. Savage, M.D. (CGB), for her project on “Telomere biology and cancer risk.”

Joseph Fraumeni (far left) and John Niederhuber (far right) with (top to bottom) Kenneth Adams and Farin Kamangar; Julie Grey, Jenna Nober, José Reyes, Patricia Chandler, Jennifer Connor, Holly Brown, Tawanda Roy, Elyse Wiszneauckas, and Cherise Banks; and Bu-Tian Ji and Mary Lou McMaster.
Three articles published in 2006 were selected for Outstanding Research Paper by a Fellow Awards in a competition judged by the Division’s Senior Advisory Group based on each paper’s impact, innovation, and clarity of thought and language. Awards were presented to Kenneth Adams, Ph.D. (NEB), for his paper on “Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old,” which was published in the New England Journal of Medicine; Farin Kamangar, M.D., M.P.H. (NEB), for his paper on “Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity,” published in the Journal of the National Cancer Institute; and Ola Landgren, M.D., Ph.D. (GEB), for his paper on “Risk of monoclonal gammopathy of undetermined significance and subsequent multiple myeloma among African American and white veterans in the United States,” published in Blood.
Awards for Outstanding Research Paper by a Staff Scientist/Clinician were given to José A. Jerónimo, M.D. (HREB), for his paper titled “Digital tools for collecting data from cervigrams for research and training in colposcopy,” which was published in the Journal of Lower Genital Tract Disease; Bu-Tian Ji, M.D., Dr.P.H. (OEEB), for his paper on “Tobacco smoking and colorectal hyperplastic and adenomatous polyps,” published in Cancer Epidemiology, Biomarkers & Prevention; and Mary Lou McMaster, M.D. (GEB), for her paper titled “Genomewide linkage screen for Waldenström macroglobulinemia susceptibility loci in high-risk families,” published in the American Journal of Human Genetics.
DCEG Fellowship Achievement Awards, which bestow a two-step Cancer Research Training Award stipend increase, were given to Jiyoung Ahn, Ph.D. (NEB), Dr. Berndt, and Anne C.M. Thiébaut, Ph.D. (NEB).
DCEG Special Recognition Awards were given to DCEG’s two outgoing Women Scientist Advisors, Lynn R. Goldin, Ph.D. (GEB), and Debra T. Silverman, Sc.D. (OEEB). The NCI Core Genotyping Facility (CGF) team involved in the CGEMS project was also recognized (Stephen J. Chanock, M.D., Xiang Deng, M.S., Amy Hutchinson, M.S., Kevin Jacobs, Marianne Rivera-Silva, Brian Staats, M.S., Gilles F. Thomas, M.D., Ph.D., Zhaoming Wang, Robert Welch, M.S., and Meredith Yeager, Ph.D.). In addition, Rochelle E. Curtis, M.A. (REB), was recognized for her accomplishment as lead editor of the recent NCI monograph titled New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000.
Sophia S. Wang, Ph.D., a tenure-track investigator in HREB, received the DCEG Outstanding Mentor Award in recognition of her exceptional commitment to the growth and productivity of junior scientists.
A DCEG Exemplary Service Award was given to Dr. Chanock for his leadership in the application of high-throughput genotyping approaches to molecular epidemiologic studies; his generous teaching through seminars, DCEG’s molecular epidemiology course, and an NCI-wide genetic analysis course; and his service on the Board of Directors for the Special Love Organization and as the Medical Director for Camp Fantastic.
Additionally, Demetrius Albanes, M.D. (NEB and Office of Education [OE]), received a DCEG Exemplary Service Award for his service as Chief of OE and as a senior investigator in NEB over the past six years. In NEB, he has led the Alpha-tocopherol Beta-carotene Cancer Prevention Cohort Study, directed important research on the role of micronutrients, and mentored fellows within the Branch. Under his watch as Chief of OE, the Division’s training program has expanded to include a wide range of new initiatives and more than 70 young investigators in training. OE has developed or supported a multitude of programs and initiatives, including the summer fellowship program; DCEG’s participation in the NIH National Graduate Student Research Festival; fellows colloquia; research ethics training; the visiting scholar program; and graduate program partnerships with universities. As he steps down as Chief of OE to devote full time to research and mentoring within NEB, the Division thanked him for his contributions.
SCIENTIFIC HIGHLIGHTS
BLADDER CANCER
DNA Repair Genes
To assess the association of common genetic variation in the base excision repair (BER) pathway with bladder cancer risk, 43 single nucleotide polymorphisms (SNPs) in 12 BER genes (OGG1, MUTYH, APEX1, PARP1, PARP3, PARP4, XRCC1, POLB, POLD1, PCNA, LIG1, and LIG3) were analyzed. Among 1,150 subjects with urinary bladder transitional cell carcinoma and 1,149 controls from the Spanish Bladder Cancer Study, SNPs in OGG1, PARP1, and POLB showed significant associations with bladder cancer risk. Subjects with a heterozygous or homozygous variant for an OGG1 SNP in the promoter region (rs125701) had significantly decreased bladder cancer risk compared with common homozygous subjects (odds ratio [OR] = 0.78; CI = 0.63–0.96). Heterozygous or homozygous individuals for the functional SNP PARP1 rs1136410 (V762A) or for the intronic SNP POLB rs3136717 were at increased risk compared with those homozygous for the common alleles, with ORs of 1.24 (CI = 1.02–1.51) and 1.30 (CI = 1.04–1.62), respectively. (Figueroa JD, Malats N, Real FX, Silverman D, Kogevinas M, Chanock S, Welch R, Dosemeci M, Tardon A, Serra C, Carrato A, García-Closas R, Castaño-Vinyals G, Rothman N, García-Closas M. Genetic variation in the base excision repair pathway and bladder cancer risk. Hum Genet 2007;121:233–242)
Large-scale Candidate Gene Study
In the same study population, an evaluation of 1,433 SNPs in 386 genes was conducted to identify common variants that influence bladder cancer risk. The most significant finding was in the 5' untranslated region (UTR) of VEGF (rs25648, p for likelihood ratio test, 2 degrees of freedom = 1 x 10-5). To further investigate the region, 29 additional SNPs in VEGF were selected and analyzed to saturate the promoter and 5' UTR and to tag common genetic variation in this gene. Three additional SNPs in the promoter region (rs833052, rs1109324, and rs1547651) were associated with increased risk of bladder cancer, with ORs of 2.52 (1.06–5.97), 2.74 (1.26–5.98), and 3.02 (1.36–6.63), respectively; a polymorphism in intron 2 (rs3024994) was associated with reduced risk (OR = 0.65; CI = 0.46–0.91). Two of the promoter SNPs and the intron 2 SNP showed linkage disequilibrium with rs25648. Haplotype analyses revealed three blocks of linkage disequilibrium, with significant associations for two blocks, including the promoter and 5' UTR (global p = 0.02 and 0.009, respectively). VEGF is critical in angiogenesis, its elevated expression in bladder tumors correlates with tumor progression, and specific 5' UTR haplotypes have been shown to influence promoter activity. (García-Closas M, Malats N, Real FX, Yeager M, Welch R, Silverman D, Kogevinas M, Dosemeci M, Figueroa J, Chatterjee N, Tardon A, Serra C, Carrato A, García-Closas R, Murta-Nascimento C, Rothman N, Chanock SJ. Large-scale evaluation of candidate genes identifies associations between VEGF polymorphisms and bladder cancer risk. PLoS Genet 2007;3:e29)
Water Disinfection Byproducts
Also from the Spanish study, the authors examined whether bladder cancer risk was associated with exposure to trihalomethanes (THMs) through ingestion of water and through inhalation and dermal absorption during showering, bathing, and swimming in pools. Lifetime personal information on water consumption and water-related habits was collected for 1,219 cases and 1,271 controls and was linked with THM levels in geographic study areas. Long-term THM exposure was associated with an OR of 2.10 (CI = 1.09–4.02) for average household THM levels of more than 49 microg/liter vs. 8 microg/liter or less. Compared with subjects not drinking chlorinated water, subjects with THM exposure of more than 35 microg/day through ingestion had an OR of 1.35 (CI = 0.92–1.99). The OR for duration of shower or bath weighted by residential THM level was 1.83 (CI = 1.17–2.87) for the highest vs. lowest quartile. Swimming in pools was associated with an OR of 1.57 (CI = 1.18–2.09). Bladder cancer risk was associated with long-term exposure to THMs in chlorinated water at levels regularly occurring in industrialized countries. (Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, García-Closas R, Serra C, Carrato A, Castaño-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M. Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 2007;165:148–156)
BRAIN TUMORS
Lead and Genetic Susceptibility
The ALAD G177C polymorphism affects the toxicokinetics of lead and may confer genetic susceptibility to its adverse effects. Occupational exposure to lead and risk of brain tumors were examined in a multisite case-control study of 489 patients with glioma, 197 with meningioma, and 799 noncancer controls. The ALAD genotype was assessed by a TaqMan assay for 355 glioma patients, 151 meningioma patients, and 505 controls. Increased risk of meningioma with occupational lead exposure (estimated by OR and CI) was most apparent in individuals with the ALAD2 variant allele, for whom risk increased from 1.1 (0.3–4.5) to 5.6 (0.7–45.5) and 12.8 (1.4–120.8) for estimated cumulative lead exposures of 1 to 49 microg/m3-y, 50 to 99 microg/m3-y, and 100 microg/m3-y or more, respectively, compared with unexposed individuals (two-sided p for trend = 0.06). This relationship became stronger after excluding occupational lead exposures characterized by a low confidence level or occurring in the 10 years before meningioma diagnosis. Occupational lead exposure was not associated with glioma risk. (Rajaraman P, Stewart PA, Samet JM, Schwartz BS, Linet MS, Zahm SH, Rothman N, Yeager M, Fine HA, Black PM, Loeffler J, Shapiro WR, Selker RG, Inskip PD. Lead, genetic susceptibility, and risk of adult brain tumors. Cancer Epidemiol Biomarkers Prev 2006;15:2514–2520)
BREAST CANCER
CYP19A1 and Estrogen Levels
Gene resequencing and a haplotype-based analysis were used to comprehensively survey common genetic variation across the CYP19A1 locus in relation to circulating postmenopausal steroid hormone levels and breast cancer risk among 5,356 breast cancer cases and 7,129 controls from five cohorts within the NCI Breast and Prostate Cancer Cohort Consortium. A high-density SNP map of 103 common SNPs (≥ 5% frequency) was used to identify linkage disequilibrium and haplotype patterns across CYP19A1, and 19 haplotype-tagging SNPs were selected to provide high predictability for the common haplotype patterns. Haplotype-tagging SNPs and common haplotypes spanning the coding and proximal 5' region of CYP19A1 were associated with a 10% to 20% increase in estrogen levels in postmenopausal women. However, no significant associations were observed between these SNPs or common haplotypes and breast cancer risk. (Haiman CA, Dossus L, Setiawan VW, Stram DO, Dunning AM, Thomas G, Thun MJ, Albanes D, Altshuler D, Ardanaz E, Boeing H, Buring J, Burtt N, Calle EE, Chanock S, Clavel-Chapelon F, Colditz GA, Cox DG, Feigelson HS, Hankinson SE, Hayes RB, Henderson BE, Hirschhorn JN, Hoover R, Hunter DJ, Kaaks R, Kolonel LN, Le Marchand L, Lenner P, Lund E, Panico S, Peeters PH, Pike MC, Riboli E, Tjonneland A, Travis R, Trichopoulos D, Wacholder S, Ziegler RG. Genetic variation at the CYP19A1 locus predicts circulating estrogen levels but not breast cancer risk in postmenopausal women. Cancer Res 2007;67:1893–1897)
Dietary Fat
The relationship between dietary fat consumption and the incidence of postmenopausal breast cancer was prospectively analyzed in the NIH-AARP Diet and Health Study, including 188,736 postmenopausal women who completed a 124-item food-frequency questionnaire between 1995 and 1996. Over an average follow-up of 4.4 years with 3,501 cases of invasive breast cancer, the hazard ratio (HR) of breast cancer for the highest (median intake = 40.1% of energy from total fat) vs. the lowest (median intake = 20.3% of energy from total fat) quintile of total fat intake was 1.11 (CI = 1.00–1.24; p for trend = 0.017). The corresponding HR for a twofold increase in percentage of energy from total fat on the continuous scale was 1.15 (CI = 1.05–1.26). Positive associations were also found for subtypes of fat (for 2-fold increase in percentage of energy from saturated fat, HR = 1.13, CI = 1.05–1.22; from monounsaturated fat, HR = 1.12, CI = 1.03–1.21; and from polyunsaturated fat, HR = 1.10, CI = 1.01–1.20). Correction for measurement error in nutrient intakes yielded an estimated HR for total fat of 1.32 (CI = 1.11–1.58). Secondary analyses showed that associations between total, saturated, and monounsaturated fat intakes were confined to women not using menopausal hormone therapy at baseline. (Thiebaut ACM, Kipnis V, Chang SC, Subar AF, Thompson FE, Rosenberg PS, Hollenbeck AR, Leitzmann M, Schatzkin A. Dietary fat and postmenopausal invasive breast cancer in the National Institutes of Health–AARP Diet and Health Study Cohort. J Natl Cancer Inst 2007;99:451–462)
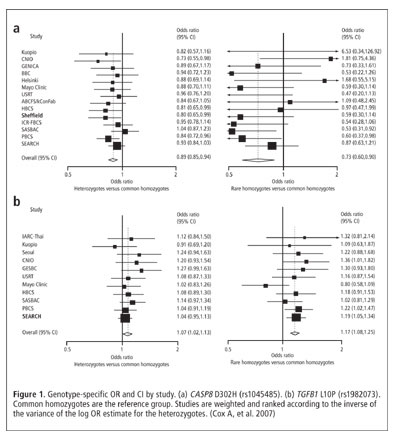
SNPs with Prior Hypotheses
The Breast Cancer Association Consortium was established to conduct combined case-control analyses with augmented statistical power to test putative genetic associations with breast cancer. Nine SNPs that had prior evidence of an association with breast cancer were genotyped: CASP8 D302H (rs1045485), IGFBP3 –202 C→A (rs2854744), SOD2 V16A (rs1799725), TGFB1 L10P (rs1982073), ATM S49C (rs1800054), ADH1B 3' UTR A→G (rs1042026), CDKN1A S31R (rs1801270), ICAM5 V301I (rs1056538), and NUMA1 A794G (rs3750913). Data from 9 to 15 studies, comprising 11,391 to 18,290 cases and 14,753 to 22,670 controls, were included. Breast cancer was associated with CASP8 D302H (for heterozygotes, OR = 0.89, CI = 0.85–0.94; for rare homozygotes, OR = 0.74, CI = 0.62–0.87; p for trend = 1.1 x 10-7) (see Figure 1). Weaker evidence was found for TGFB1 L10P (for heterozygotes, OR = 1.07, CI = 1.02–1.13; for rare homozygotes, OR = 1.16, CI = 1.08–1.25; p for trend = 2.8 x 10-5). Results demonstrate that common breast cancer susceptibility alleles with small effects on risk can be identified, given sufficiently powerful studies. (Cox A, Dunning AM, García-Closas M, et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat Genet 2007;39:352–358)
Seminar For Science Writers Discusses Risks For Second Cancers

In January, NCI sponsored a Science Writers Seminar on Second Cancers to coincide with the publication of the NCI monograph New Malignancies among Cancer Survivors: SEER Cancer Registries, 1973–2000. Opening the seminar, Lois B. Travis, M.D., Sc.D., of the Radiation Epidemiology Branch (REB), introduced the topic and reviewed epidemiologic methods to assess second cancer risk. She discussed a DCEG study indicating an increased breast cancer risk following treatment for Hodgkin lymphoma.
Rochelle E. Curtis, M.A. (REB), the monograph’s lead editor, presented an overview of the risk of new malignancies among the more than two million cancer survivors featured in the publication, including detailed data on 50 adult and 18 childhood cancer sites. Ms. Curtis highlighted selected results, including the radiation-related risk seen among childhood cancer survivors and notably high excesses associated with tobacco- and alcohol-related cancers among women and men.
Margaret A. Tucker, M.D., Director of the Human Genetics Program, Chief of the Genetic Epidemiology Branch, and a monograph coeditor, reviewed the effects of genetic susceptibility on second cancer risk. She cited the DCEG study of survivors with heritable retinoblastoma who are prone to develop excess sarcomas, melanoma, and cancers of the brain and nasal cavities, with an even higher risk following radiotherapy.
The seminar webcast allowed for wide coverage to hundreds of Internet viewers. The monograph is available online.
—Rochelle E. Curtis, M.A.
COLORECTAL ADENOMAS
Base Excision Repair Genes
To examine the relationship between genetic variation in BER genes and colorectal adenoma risk, a case-control study of 767 advanced colorectal adenoma cases and 773 controls from the baseline screening exam of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial was conducted. Cases included participants diagnosed with advanced left-sided adenoma; controls had no evidence of a left-sided polyp by sigmoidoscopy. Among 20 SNPs in four BER genes (APEX1, PARP1, POLB, and XRCC1) genotyped, two variants with possible functional significance were associated with risk. The APEX1 51H variant was associated with a borderline significant decreased risk of colorectal adenoma (OR = 0.66; CI = 0.44–1.00), and the XRCC1 399Q variant was inversely associated with risk among whites (OR = 0.80; CI = 0.64–0.99). Homozygotes at two PARP1 loci (A284A and IVS13+118G→A) were also associated with a decreased risk of colorectal adenoma compared with wild-type carriers (OR = 0.70; CI = 0.49–0.98 for both), which was restricted to advanced adenomas displaying histologically aggressive characteristics (OR = 0.51; CI = 0.33–0.78; p = 0.002 for PARP1 A284A). (Berndt SI, Huang WY, Fallin MD, Helzlsouer KJ, Platz EA, Weissfeld JL, Church TR, Welch R, Chanock SJ, Hayes RB. Genetic variation in base excision repair genes and the prevalence of advanced colorectal adenoma. Cancer Res 2007;67:1395–1404)
ENDOMETRIAL CANCER
Lifetime Body Weight
The joint effects of lifetime body weight and menopausal hormone therapy (MHT) on endometrial cancer risk were assessed in a cohort of 103,882 women aged 50 to 71 years in 1995–1996. During a median of 4.6 years and 455,304 person-years of follow-up through 2000, both baseline body mass index (BMI) and adult weight gain were associated with increased endometrial cancer risk. The multivariate relative risk (RR) comparing obese with normal weight women (BMI > 30 vs. < 25 kg/m2) was 3.03 (CI = 2.50–3.68). Compared with women with stable weight (gained or lost < 5 kg) between age 18 and baseline, women who gained 20 kg or more had an RR of 2.75 (CI = 1.96–3.86). MHT significantly modified the relationships of BMI (p for interaction < 0.001) and adult weight gain (p for interaction = 0.004) to risk. Compared with normal weight, the RRs (CI) for obesity were 5.41 (4.01–7.29) among women who never used MHT, 2.53 (1.21–5.30) among former MHT users, and 1.44 (1.00–2.05) among current users. Compared with a stable weight between age 18 and baseline, the RRs (CI) for weight gain of at least 20 kg among never users and ever users of MHT were 5.35 (3.01–9.52) and 1.43 (0.96–2.15), respectively. (Chang SC, Lacey JV Jr, Brinton LA, Hartge P, Adams K, Mouw T, Carroll L, Hollenbeck A, Schatzkin A, Leitzmann MF. Lifetime weight history and endometrial cancer risk by type of menopausal hormone use in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev 2007;16:723–730)
LEUKEMIA AND LYMPHOMA
Hairy Cell Leukemia and Second Cancers
Second cancer incidence and cause-specific mortality were quantified among 3,104 survivors of hairy cell leukemia reported to 16 registries in the Surveillance, Epidemiology, and End Results (SEER) Program between 1973 and 2002. With a mean follow-up of 6.5 years, second cancer risk was elevated (standardized incidence ratio [SIR] = 1.24; CI = 1.11–1.37) compared with the general population. Survivors had higher risks of Hodgkin lymphoma (HL) (SIR = 6.61; CI = 2.13–15.42), non-Hodgkin lymphoma (NHL) (SIR = 5.03; CI = 3.77–6.58), and thyroid cancer (SIR = 3.56; CI = 1.30–7.74) and a lower risk of lung cancer (SIR = 0.63; CI = 0.42–0.90). The cumulative probability of all second cancers was estimated to be 31.9% (CI = 26.2–37.6) at 25 years after hairy cell leukemia diagnosis. Among 10,000 hairy cell leukemia patients, an excess of about 34 cancers, including 21 NHL, 2 HL, and 7 solid tumors (including 2 thyroid cancers), might be observed per year. Deaths due to solid tumors were not elevated compared with the general population (standardized mortality ratio [SMR] = 0.9), and there were deficits in mortality due to both cardiovascular (SMR = 0.67; CI = 0.56–0.80) and cerebrovascular (SMR = 0.61; CI = 0.38–0.93) disease. (Hisada M, Chen BE, Jaffe ES, Travis LB. Second cancer incidence and cause-specific mortality among 3,104 patients with hairy cell leukemia: a population-based study. J Natl Cancer Inst 2007;99:215–222)
Genetic Susceptibility to Chronic Lymphocytic Leukemia (CLL)
To further investigate a genome scan finding, the authors selected six CLL families comprising 63 individuals (19 with CLL, 44 unaffected) for fine mapping of a 23-megabase region in 13q14.2-q22.2. Interphase fluorescence in situ hybridization (FISH) revealed 13q14 deletion in 85% (11/13) of CLL patients. Four CLL families shared a 3.68 Mb minimal region in 13q21.33-q22.2. Two asymptomatic siblings who shared the 13q21.33-q22.2 at-risk haplotype exhibited CD5+ monoclonal B-cell lymphocytosis (MBL) on flow cytometry. One of these individuals also had a 13q14 deletion by FISH. These two individuals with MBL shared the at-risk haplotype with their CLL-affected relatives, providing further evidence of the relationship between CLL and MBL and of the biologic significance of this region. Using direct DNA sequencing analysis, 13 genes were screened for mutations, but no frameshift or nonsense mutations were detected. Eleven of the 13 genes in the candidate region were expressed in immune tissues, supporting their functional relevance in investigations of familial CLL. (Ng D, Toure O, Wei MH, Arthur DC, Abbasi F, Fontaine L, Marti GE, Fraumeni JF Jr, Goldin LR, Caporaso N, Toro JR. Identification of a novel chromosome region, 13q21.33-q22.2, for susceptibility genes in familial chronic lymphocytic leukemia. Blood 2007;109:916–925)
Immune Gene Polymorphisms and Lymphoma Prognosis
The hypothesis that common germline variation in candidate immune genes is associated with follicular lymphoma survival was evaluated using Cox models to estimate adjusted HRs for individual SNPs. The median age at diagnosis of the 278 patients was 57 years, and 59 (21%) of the patients died during follow-up, with a median follow-up of 59 months (range = 27 to 78 months) for surviving patients. SNPs in IL8 (rs4073; HRTT = 2.14, CI = 1.26–3.63), IL2 (rs2069762; HRGT/TT = 1.80, CI = 1.06–3.05), IL12B (rs3212227; HRAC/CC = 1.83, CI = 1.06–3.06), and IL1RN (rs454078; HRAA = 1.93, CI = 1.11–3.34) were the most robust predictors of survival. A summary score of the number of deleterious genotypes from these genes was strongly associated with survival (p = 0.001). A risk score that combined the four SNPs with clinical and demographic factors was even more strongly associated with survival (p = 1.8 x 10-11); the five-year Kaplan-Meier survival estimates were 96% (93%–100%), 72% (62%–83%), and 58% (48%–72%) for low-, intermediate-, and high-risk groups, respectively. Common variation in host immune genes warrants further evaluation as a promising class of prognostic factors in follicular lymphoma. (Cerhan JR, Wang S, Maurer MJ, Ansell SM, Geyer SM, Cozen W, Morton LM, Davis S, Severson RK, Rothman N, Lynch CF, Wacholder S, Chanock SJ, Habermann TM, Hartge P. Prognostic significance of host immune gene polymorphisms in follicular lymphoma survival. Blood 2007;109:5439–5446)
Cancer Risk after HL
Among 18,862 five-year HL survivors from 13 population-based cancer registries in North America and Europe, Poisson regression was used to evaluate the effects of age at diagnosis, attained age, latency, sex, treatment, and year of diagnosis on the RR and excess absolute risk (EAR) of solid cancers (SC). Of 1,490 identified SC, 850 were estimated to be in excess. For most cancer sites, both RR and EAR decreased with age at HL diagnosis and showed strong dependencies on attained age. For a patient diagnosed at age 30 years who survived to at least 40 years, modeled risks were significantly elevated for cancers of the breast (RR = 6.1), other supradiaphragmatic sites (RR = 6.0), and infradiaphragmatic sites (RR = 3.7); the largest RR (20-fold) was observed for malignant mesothelioma. Thirty-year cumulative risks of SC for men and women diagnosed at 30 years were 18% and 26%, respectively, compared with 7% and 9% in the general population. For young HL patients, risks of breast and colorectal cancers were elevated 10 to 25 years before the age when routine screening would be recommended in the general population. (Hodgson DC, Gilbert ES, Dores GM, Schonfeld SJ, Lynch CF, Storm H, Hall P, Langmark F, Pukkala E, Andersson M, Kaijser M, Joensuu H, Fossa SD, Travis LB. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol 2007;25:1489–1497)
Family History and NHL
A pooled analysis of 10,211 NHL cases and 11,905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) was conducted to evaluate NHL risk among those with hematopoietic malignancies in first-degree relatives. NHL risk was elevated for individuals who reported first-degree relatives with NHL (OR = 1.5; CI = 1.2–1.9), HL (OR = 1.6; CI = 1.1–2.3), and leukemia (OR = 1.4; CI = 1.2–2.7). Risk was highest among individuals who reported a brother with NHL (OR = 2.8; CI = 1.6–4.8) and was consistent for all NHL subtypes evaluated. If a first-degree relative had HL, NHL risk was highest if the relative was a parent (OR = 1.7; CI = 1.0–2.9). If a first-degree relative had leukemia, NHL risk was highest among women who reported a sister with leukemia (OR = 3.0; CI = 1.6–5.6). The pattern of NHL heritability appeared to be uniform across NHL subtypes, but risk patterns differed by specific hematopoietic malignancies and the sex of the relative. (Wang SS, Slager SL, Brennan P, Holly EA, De Sanjose S, Bernstein L, Boffetta P, Cerhan JR, Maynadie M, Spinelli JJ, Chiu BC, Cocco P, Mensah F, Zhang Y, Nieters A, Dal Maso L, Bracci PM, Costantini AS, Vineis P, Severson RK, Roman E, Cozen W, Weisenburger D, Davis S, Franceschi S, La Vecchia C, Foretova L, Becker N, Staines A, Vornanen M, Zheng T, Hartge P. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma: A pooled analysis of 10,211 cases and 11,905 controls from the InterLymph Consortium. Blood 2007;109:3479–3488)
Gene-nutrient Interactions and NHL
The main effects on NHL of 30 polymorphisms in 18 genes involved in one-carbon metabolism or DNA repair were investigated among 1,141 incident cases and 949 population-based controls. Gene-nutrient interactions were examined in a subgroup of 386 cases and 319 controls with food-frequency information. Decreased risk of NHL was observed with BHMT Ex8+453A→T and increased risk with CBS Ex13+41C→T, FPGS Ex15-263T→C, and SHMT1 Ex12+138C→T and Ex12+236C→T. Significant gene-nutrient interactions limited a protective association comparing high vs. low vitamin B6 to FPGS Ex15-263T→C CC (OR = 0.22; CI = 0.10–0.52), MTHFS IVS2-1411T→G TT/TG (OR = 0.54; CI = 0.36–0.81), and MTR Ex26-20A→G AA (OR = 0.55; CI = 0.35–0.86) genotypes and a protective association of methionine to FTHFD Ex10-40G→T GG (OR = 0.63; CI = 0.44–0.91), MTHFR Ex8-62A→C CC (OR = 0.13; CI = 0.04–0.39), and MTRR Ex5+136T→C TT (OR = 0.67; CI = 0.47–0.97) genotypes. (Lim U, Wang SS, Hartge P, Cozen W, Kelemen LE, Chanock S, Davis S, Blair A, Schenk M, Rothman N, Lan Q. Gene-nutrient interactions among determinants of folate and one-carbon metabolism on the risk of non-Hodgkin lymphoma: NCI-SEER case-control study. Blood 2007;109:3050–3059)
Oxidative Stress Pathway and NHL
In a population-based case-control study of NHL (n = 518 cases, 597 controls) among women in Connecticut, SNPs in 10 candidate genes (AKR1A1, AKR1C1, AKR1C3, CYBA, GPX1, MPO, NOS2A, NOS3, OGG1, and SOD2) that directly or indirectly mediate oxidative stress in the NADPH oxidase-dependent respiratory burst were analyzed. There was a 1.7-fold (CI = 1.2–2.4; p = 0.0047) increased risk of NHL for individuals who were variant homozygous for the AKR1A1 (IVS5+282T→C) SNP. The effect was most pronounced for risk of diffuse large B-cell lymphoma, but risk estimates were also non-significantly elevated for other common B-cell histologies and T-cell lymphomas. Individuals variant homozygous for the CYBA (Ex4+11C→T) SNP had a 1.6-fold (CI = 1.1–2.4; p = 0.019) increased risk of NHL that was pronounced for T-cell lymphoma (OR = 3.5; CI = 1.3–9.6; p = 0.013) but was also suggestively associated with each of the common B-cell histologies. (Lan Q, Zheng T, Shen M, Zhang Y, Wang SS, Zahm SH, Holford TR, Leaderer B, Boyle P, Chanock S. Genetic polymorphisms in the oxidative stress pathway and susceptibility to non-Hodgkin lymphoma. Hum Genet 2007;121:161–168)
LIVER CANCER
Medical Conditions and Cholangiocarcinoma
This nationwide population-based case-control study in Denmark examined relationships between selected medical conditions and later intrahepatic cholangiocarcinoma (ICC) risk among 764 cases diagnosed between 1978 and 1991 and 3,056 population controls, who were linked to the Danish hospital discharge registry to obtain information on prior hospital diagnoses. Alcoholic liver disease and unspecified cirrhosis were associated with ICC, with ORs of 19.22 (CI = 5.55–66.54) and 75.9 (CI = 10.2–565.7), respectively. Cholangitis (OR = 6.3; CI = 2.3–17.5), choledocholithiasis (OR = 23.97; CI = 2.9–198.9), and cholecystolithiasis (OR = 4.0; CI = 2.0–7.99) were also associated with elevated risk, but gallbladder removal did not significantly change risk (OR = 1.6; CI = 0.65–3.7). Chronic inflammatory bowel disease (OR = 4.7; CI = 1.65–13.9) was associated with ICC, and diabetes was associated with risk in the year prior to ICC diagnosis (OR = 3.02; CI = 1.05–8.69). Obesity was unrelated to risk. Results confirm that prior bile duct diseases increase risk of ICC and suggest that alcoholic liver disease and diabetes may also increase risk. (Welzel TM, Mellemkjaer L, Gridley G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: A nationwide case-control study. Int J Cancer 2007;120:638–641)
LUNG CANCER
Risk among AIDS Patients
Lung cancer risk was compared between people with AIDS (PWA) and the general population, and its relationship with immunosuppression was assessed. Records on adolescent and adult PWA (n = 397,927) were linked with cancer registries in 11 U.S. regions. Cancer risk was assessed for the period 60 months before to 60 months after AIDS onset, with specific emphasis on the period 4 to 27 months after onset. Compared with the general population, lung cancer risk among PWA was elevated overall (n = 1,489 cases; SIR = 3.8; CI = 3.6–4.1) and in the 4 to 27 months after AIDS onset (SIR = 2.9; CI = 2.6–3.2). In the 4 to 27 months after AIDS onset, risk was elevated for all demographic subgroups and was especially high among young PWA (SIRs for ages 15–29 years = 10.4; 30–39 years = 6.3; 40–49 years = 3.7). Lung cancers generally presented at an advanced stage. Risk was not associated with CD4 cell counts at AIDS onset. Under plausible smoking assumptions, observed incidence was higher than predicted among 40–49- and 50–59-year-old men with AIDS (observed/predicted = 5.03 and 1.43, respectively) and 40–49-year-old women with AIDS (observed/predicted = 1.88), but not among older PWA. (Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS 2007;21:207–213)
MELANOMA
Predictors of Germline CDKN2A Mutations
Several factors, namely an increased number of patients with melanoma in a family, early age at melanoma diagnosis, and family members with multiple primary melanomas (MPM) or pancreatic cancer, were examined in relation to CDKN2A mutation frequency in 385 families with 3 or more patients with melanoma pooled into 17 GenoMEL groups and compared across continents. Overall, 39% of families had CDKN2A mutations ranging from 20% (32/162) in Australia to 45% (29/65) in North America to 57% (89/157) in Europe. All four factors in each group, except pancreatic cancer in Australia (p = 0.38), individually showed significant associations with CDKN2A mutations, but effects varied widely across continents. Multivariate examination also showed different predictors of mutation risk across continents. In Australian families, having at least two patients with MPM, median age at melanoma diagnosis by age 40 years, and six or more patients with melanoma in a family jointly predicted the mutation risk. In European families, all four factors concurrently predicted the risk, but with less stringent criteria than in Australia. In North American families, only having at least one patient with MPM and diagnosis by age 40 years simultaneously predicted the mutation risk. (Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Timothy BD, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Cannon Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Teresa LM, Lang J, Leachman SA, Mackie RM, Magnusson V, Mann GJ, Newton BJ, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet 2007;44:99–106)
METHODS
Powerful Multilocus Tests
In genetic epidemiology studies, the association between a disease and a genomic region, such as a candidate gene, is often investigated using multiple SNPs. On the basis of Tukey’s model, the authors propose a novel but computationally simple generalized test of association that can simultaneously capture both the main effects of the variants within a genomic region and their interactions with the variants in another region or with an environmental exposure. Performance of this method and that of two standard tests of association, one ignoring gene-gene/gene-environment interactions and the other based on a saturated model of interactions, were compared. Major power advantages of the novel method were demonstrated in analysis of data from a case-control study of the association between colorectal adenoma and DNA variants in the NAT2 genomic region, which are known to be related to a common biological phenotype, and under different models of gene-gene interactions with simulated data. (Chatterjee N, Kalaylioglu Z, Moslehi R, Peters U, Wacholder S. Powerful multilocus tests of genetic association in the presence of gene-gene and gene-environment interactions. Am J Hum Genet 2006;79:1002–1016)
PROSTATE CANCER
Second Risk Locus at 8q24
Recently, common variants on human chromosome 8q24 were found to be associated with prostate cancer risk. While conducting a genome-wide association study in the Cancer Genetic Markers of Susceptibility project with 550,000 SNPs in a nested case-control study (n = 1,172 cases and 1,157 controls of European origin), a new association at 8q24 with an independent effect on prostate cancer susceptibility was identified. The most significant signal is 70 kb centromeric to the previously reported SNP, rs1447295, but shows little evidence of linkage disequilibrium with it. A combined analysis with four additional studies (n = 4,296 cases and 4,299 controls) confirms association with prostate cancer for rs6983267 in the centromeric locus (p = 9.42 x 10-13; heterozygote OR = 1.26, CI = 1.13–1.41; homozygote OR = 1.58, CI = 1.40–1.78) (see Figure 2). Each SNP remained significant in a joint analysis after adjusting for the other (rs1447295, p = 1.41 x 10-11; rs6983267, p = 6.62 x 10-10). These observations, combined with compelling evidence for a recombination hotspot between the two markers, indicate the presence of at least two independent loci within 8q24 that contribute to prostate cancer in men of European ancestry. The population attributable risk of the new locus, marked by rs6983267, is estimated to be higher than that of the locus marked by rs1447295 (21% vs. 9%). (Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet 2007;39:645–649)
DCEG PEOPLE IN THE NEWS

Anil Chaturvedi
In December, CAPT Linda Morris Brown, Dr.P.H., Biostatistics Branch (BB), was selected as chair of the Research Functional Group, an advisory committee created by the Commissioned Corps to aid research officers in their career advancement.
In February, Nilanjan Chatterjee, Ph.D. (BB), gave an invited talk titled “Inference in studies of genes and environments: Novel methods” at the Statistical Methods for Gene-environment Interaction Workshop at the International Agency for Research for Cancer in Lyon, France.
Anil K. Chaturvedi, Ph.D., Viral Epidemiology Branch (VEB), was selected to receive the American Society of Clinical Oncology (ASCO) Merit Award, including a $1,500 stipend for travel to the 2007 ASCO Annual Meeting in Chicago, for his abstract titled “Incidence trends for human papillomavirus-related and -unrelated head and neck squamous cell carcinomas in the U.S.”
In March, Eric A. Engels, M.D., M.P.H. (VEB), gave an invited talk titled “Epidemiologic approaches to identify cancers with an infectious etiology” at the Fred Hutchinson Cancer Research Center in Seattle.

Jonine Figueroa
In January, Jonine D. Figueroa, Ph.D., M.P.H., Hormonal and Reproductive Epidemiology Branch (HREB), was awarded a Keystone Symposia Minority Scholarship to attend its meeting on genome instability and repair in Breckenridge, Colorado and to present her abstract titled “Evaluation of genetic variation in the double-strand break repair pathway suggests an association with bladder cancer risk.”
Mitchell H. Gail, M.D., Ph.D. (Chief of BB), delivered a lecture titled “Absolute risk: Clinical applications and controversies” at the Department of Statistics of Columbia University in New York City in March. He also gave talks on “Absolute risk: Estimation and applications” at NCI’s Statistical Research and Applications Branch in the Division of Cancer Control and Population Sciences in January and at NCI’s Laboratory of Human Carcinogenesis in the Center for Cancer Research in February.
Alisa M. Goldstein, Ph.D., Genetic Epidemiology Branch (GEB), gave an invited talk titled “p16 mutations in melanoma: A 2007 perspective” at the Fourth International Symposium on Melanoma and Other Cutaneous Malignancies in New York City in March.
In April, Harry Haverkos, M.D. (VEB), gave invited talks on “Kaposi’s sarcoma: HHV-8, immunosuppression, and vasoactive agents” at the University of Cincinnati and at Emory University in Atlanta.
 Aaron Blair
Aaron Blair
DIsTInGuIsheD alumni awarD
In April, Aaron E. Blair, Ph.D., Occupational and
Environmental Epidemiology Branch (OEEB), was
the recipient of the Harriet Hylton Barr Distinguished
Alumni Award of the University of North Carolina
(UNC) School of Public Health (SPH). Established in
1975 as the school’s single highest alumni honor, the
award recognizes the achievements of alumni and their
contributions to public health, including leadership,
experimentation, collaboration, and innovation within
the profession; impact within the practice arena; and
outstanding service beyond the requirements of the recipient’s employment. Dr. Blair was a 1976
graduate of UNC SPH.
Ann W. Hsing, Ph.D. (HREB),
presented data on polymorphisms in
inflammation genes and biliary tract
cancer from the Shanghai Biliary Tract
Cancer Study at the American Epidemiological
Society Meeting in Boston
in March.
Farin Kamangar, M.D., Ph.D., Nutritional Epidemiology Branch (NEB), recently gave invited presentations titled “Polycyclic aromatic hydrocarbons in esophageal carcinogenesis: Data from China, Iran, and Brazil” at the Johns Hopkins Bloomberg School of Public Health and Drexel University School of Public Health.
Ola Landgren, M.D., Ph.D. (GEB), gave an invited talk titled “On the pathway to multiple myeloma: New etiologic clues from epidemiological studies” at the Mayo Clinic in February.
In February, Linda Littlejohn, Administrative Resource Center, was selected for an Administrative Officer position. She has been with NCI for 19 years and with DCEG since its inception. She will support BB and the Office of the Director of the Epidemiology and Biostatistics Program.
Sheng Luo, Ph.D. (BB), won several awards this spring. He received the Distinguished Student Paper Award at the International Biometric Society’s Eastern North American Region Meeting in Atlanta. He was recently named the overall winner, as well as the winner in applied research, of the 2007 Delta Omega Scientific Poster Competition sponsored by the Johns Hopkins Bloomberg School of Public Health; his poster was titled “Analysis of smoking cessation patterns using a stochastic mixed effects model with a latent cured state.” He received the 2007 Young Investigator Award for his dissertation work from the American Statistical Association and was one of two winners of the Louis I. and Thomas D. Dublin Award for the Advancement of Epidemiology and Biostatistics from Johns Hopkins. He also received a Laha Travel Award from the Institute of Mathematical Statistics (IMS) for the Joint Statistical Meetings/IMS Meeting.
Katherine McGlynn, Ph.D., M.P.H. (HREB), gave a talk on “The epidemiology of testicular germ cell tumors” for the Yale University Epidemiology Lecture Series in January.
In December, Panagiota N. Mitrou, M.Sc., Ph.D. (NEB), gave a talk titled “Adherence to a Mediterranean dietary pattern in relation to mortality and cancer incidence in the NIH-AARP Diet and Health Study” at Imperial College in London and at the Medical Research Council’s Centre for Nutrition and Cancer Prevention and Survival at the University of Cambridge.
Ruth M. Pfeiffer, Ph.D. (BB), delivered a talk titled “A model-free approach to combining diagnostic markers” at the National Heart, Lung, and Blood Institute’s Office of Biostatistics Seminar in February. She also gave an invited talk on “Development of a colorectal cancer risk assessment tool” at the International Biometric Society’s Eastern North American Region Meeting in Atlanta in March. Preetha Rajaraman, Ph.D., Radiation
Preetha Rajaraman, Ph.D., Radiation Epidemiology Branch, gave an invited talk on “Recent findings from the NCI Adult Brain Tumor Study” at the University of York in March.
In March, Rashmi Sinha, Ph.D. (NEB), gave two talks on the “Feasibility of conducting a multicenter prospective study in India and other ongoing efforts” at Imperial College in London and at the London School of Hygiene and Tropical Medicine.
Philip R. Taylor, M.D., Sc.D. (GEB), gave an invited talk on “Esophageal cancer: Epidemiology and prevention” at the George Washington University School of Public Health and Health Services in February.
Jorge R. Toro, M.D. (GEB), recently gave talks on “Lung cyst, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome” at the American Society of Human Genetics Meeting in New Orleans in October and “Genodermatoses” at the 65th American Academy of Dermatology Meeting in Washington, DC in February.
In February, Shelia Hoar Zahm, Sc.D., DCEG Deputy Director, served as a member of an advisory panel for the European Commission Public Health Program on the “Development of public health indicators for reporting environmental/occupational risks related to agriculture and fishery” in Vougliameni, Greece.

Gabriella Andreotti, Steve Moore, and Ying-Ying Yu.
In March, three of DCEG’s predoctoral fellows successfully defended their doctoral dissertations. Gabriella Andreotti, Ph.D. (HREB), became a postdoctoral fellow in OEEB after receiving her Ph.D. from George Washington University, where she examined the effects of variants of genes in the lipid metabolism pathway and the effects of serum lipid levels on biliary tract cancer and stones. Her mentors were Ann W. Hsing, Ph.D. (HREB), and Dr. Paul Levine. Steve C. Moore, Ph.D. (NEB), completed his Ph.D. on adiposity and its relationship to mortality and cancer at Yale University under the guidance of Michael F. Leitzmann, M.D., Dr.P.H. (NEB), and Dr. Susan Mayne. He will become a postdoctoral fellow in NEB. While at the University of Maryland, Baltimore, Ying-Ying Yu, Ph.D. (VEB), investigated the role of tuberculosis and scarring in promoting the development of lung cancer under the mentorship of Eric A. Engels, M.D., M.P.H. (VEB), and Dr. Paul Pinsky. She will be joining the Epidemic Intelligence Service at the Centers for Disease Control and Prevention.
COMINGS . . . GOINGS

Houda Boukheris
Houda Boukheris, M.D., Ph.D., joined the Radiation Epidemiology Branch (REB) as an Oak Ridge Institute for Science and Education Fellow. In 1993, she received an M.D. from the Ministry of Higher Education and Scientific Research of Algeria and, in 2003, a Ph.D. in public health from the University of Algiers School of Medicine. She continued her training as an International Agency for Research on Cancer Fellow at the Institute for Health and Medical Research (INSERM) in France. While in INSERM, she worked on long-term mortality after breast cancer and prognostic factors among patients with thyroid and endocrine tumors. In REB, she will work on projects related to the Childhood Cancer Survivor Study in collaboration with Martha S. Linet, M.D., M.P.H., and Rochelle E. Curtis, M.A.

Victoria Chia
Victoria M. Chia, Ph.D., joined the Hormonal and Reproductive Epidemiology Branch (HREB) as a 2007 recipient of a Sallie Rosen Kaplan Fellowship. She received an A.B. in molecular and cell biology from the University of California, Berkeley and an M.P.H. from Emory University. While in Atlanta, she also served as a research coordinator at the American Cancer Society. In 2006, she earned a Ph.D. in epidemiology from the University of Washington, Seattle. Her dissertation focused on leptin, insulin-like growth factors, and risk of colorectal adenoma. While in HREB, she will work with Katherine McGlynn, Ph.D., M.P.H., on testicular cancer and James V. Lacey, Jr., Ph.D., on studies of endometrial and ovarian cancers.
 Thomas Fears (Photograph Credit: David Check)
Thomas Fears (Photograph Credit: David Check)
In March, Thomas R. Fears, Ph.D., retired from the Biostatistics Branch (BB). He received a Ph.D. in statistics from Iowa State University in 1972 and joined NCI in 1973 as a senior staff fellow. He has been a mathematical statistician in the Epidemiology and Biostatistics Program (EBP) since 1979. His research focused on the etiology of skin cancer, particularly the relationship of skin cancer risk and solar radiation. An early analysis highlighted the hours of the day with highest levels of UVB. Another paper comparing the incidence patterns for non-melanoma and melanoma skin cancers first proposed the “intermittent exposure” hypothesis for melanoma. He was a coinvestigator with Dr. Joseph Scotto on a special skin cancer survey to estimate the relationship between non-melanoma skin cancer rates and solar ultraviolet rates. Results were instrumental in obtaining regulatory action on chlorofluorocarbons worldwide. More recently, he worked with Margaret A. Tucker, M.D., on a melanoma case-control study. In one particularly important analysis, lifetime residential history was coupled with levels of midrange ultraviolet radiation to provide an estimate of an individual’s sunlight exposure, a measure that has been particularly difficult to quantify. In another study, a melanoma prediction model was developed for use during routine physical exams by primary care providers to identify individuals at high absolute risk, perhaps leading to detection of early, curable disease or to a decrease in an individual’s risk of developing it. The melanoma risk assessment tool can be found on the NCI web site. Dr. Fears is a recipient of the NIH Merit Award, an Equal Employment Opportunity Special Achievement Award, and the Snedecor Award from the Committee of Presidents of Statistical Societies. He will continue to work part-time as a special volunteer in the Human Genetics Program.
Gabriel Chodick, Ph.D., a visiting fellow in REB, has taken a position at the Maccabi Institute for Research in Healthcare Services of the Tel Aviv University School of Public Health.

Michael Cook
Michael B. Cook, Ph.D., joined HREB as a postdoctoral fellow. In 2003, he received his undergraduate degree in genetics from the University of Nottingham in the United Kingdom. In 2007, he received his Ph.D. in epidemiology from the University of Leeds. For his dissertation, he investigated sex differences in the risk of esophageal adenocarcinoma and its precursor lesion, Barrett’s esophagus. He is working with Dr. McGlynn on the epidemiology and etiology of testicular germ cell tumors.

Shahinaz Gadalla
Shahinaz Gadalla, M.D., M.S., joined the Viral Epidemiology Branch (VEB) as a research fellow. In 1996, she earned an M.D. from Ain Shams University, Cairo, and in 2005, an M.S. in epidemiology and preventive medicine from the University of Maryland, Baltimore, where she is currently a Ph.D. candidate in the epidemiology program. During her fellowship in VEB, she will work on the association between immune dysregulation and breast cancer risk under the mentorship of James J. Goedert, M.D.

Mercy Guech-Ongey
Mercy Guech-Ongey, Ph.D., joined VEB as a visiting fellow. Originally from Cameroon, she obtained a B.Sc. in medical laboratory technology in 1996 from the University of Calabar, Nigeria. In 2001, she obtained an M.Sc. in biotechnology from Hochschule Mannheim–University of Applied Sciences in Germany. She received her Ph.D. in epidemiology in 2005 from the University of Heidelberg, Germany. Her dissertation focused on the interactions of infections, particularly hepatitis A and Helicobacter pylori, diabetes mellitus, and serum markers of immunologic activation on the risk of cardiovascular disease. While in VEB, her primary mentor is Sam M. Mbulaiteye, M.D.

Kristine Johnson
Kristine Johnson, M.D., D.T.M.&H., joined VEB as a special volunteer. In 1996, she received a B.S. in chemistry from the University of North Carolina at Chapel Hill and, in 2001, an M.D. from Duke University School of Medicine. In 2004, she earned a Diploma of Tropical Medicine and Hygiene from the London School of Hygiene and Tropical Medicine. She then completed her internal medicine residency at the Denver medical campus of the University of Colorado and is currently a fellow in infectious disease at Johns Hopkins University. She is pursuing a Ph.D. in clinical investigation at the Johns Hopkins Bloomberg School of Public Health. Her thesis focuses on the role of circumcision and HIV in male HPV infection. As a member of VEB, her mentor is Charles S. Rabkin, M.D.

Kwang Pyo Kim
Kwang Pyo Kim, Ph.D., joined REB as a research fellow. He received his Ph.D. in nuclear and radiological engineering at the University of Florida. His doctoral dissertation assessed radiation exposure of workers in the Florida phosphate industry due to inhalation of airborne particulates containing radioactive materials. He is working on various epidemiological studies of environmental, occupational, and medical radiation exposures, including the U.S. Radiologic Technologists Study with Michele M. Doody, M.S., Alice J. Sigurdson, Ph.D., and Steven l. Simon, Ph.D., a study of interventional fluoroscopists with Ruth A. Kleinerman, M.P.H., and Martha S. Linet, M.D., M.P.H., and second cancer studies and the Medical Countermeasures against Radiological and Nuclear Threats with Andre Bouville, Ph.D., Dr. Simon, and Dr. Linet.

Lois Travis with Martha Linet (Photograph Credit: Annelie Landgren)
After 18 years with NIH, Lois B. Travis, M.D., Sc.D., retired from the PHS in May. She joined NCI in 1991 as a lead research investigator in EBP. In 1994, she was awarded scientific tenure. During her time at NIH, she established a reputation as a leading expert on second cancers due to radiation and chemotherapy treatment. The findings of her research studies on breast cancer following Hodgkin lymphoma led to changes in treatment, and she was the first to show a dose-dependent relationship between platinum-based chemotherapy and secondary leukemia risk. She also identified risk of breast cancer following radiation and chemotherapy for women with Hodgkin lymphoma and was able to quantify future risk and suggest treatment options based on the risk of secondary cancer. She pioneered international collaborative studies in Canada, Denmark, Finland, Sweden, Norway, Japan, China, France, and the Netherlands, in addition to the United States. She has taken a position as a principal scientist at Exponent, Inc., in New York City.
Stefan Lönn, Ph.D., has left REB for a teaching and research position at the Karolinska Institute in Sweden.

Gwen Murphy
Gwen Murphy, Ph.D., joined VEB as a DCP Cancer Research Training Fellow. She received an M.P.H. from the School of Public Health and Population Sciences, University College Dublin, Ireland and a Ph.D. from the Cancer Prevention Institute, Adelaide and Meath Hospital, Trinity College, Dublin. Her dissertation clarified the role of polymorphisms in cytokine and innate immunity genes in the etiology of gastric cancer. In addition to a study of diet, genetics, and inflammation in the Polyp Prevention Trial, she will work with Drs. Mbulaiteye and Rabkin on studies of Th1/Th2 and other immunologic perturbations in gastric cancer.
Margaret E. Wright, Ph.D., has left the Nutritional Epidemiology Branch to join the University of Illinois at Chicago College of Medicine as an assistant professor in the Department of Pathology and an adjunct professor in the Department of Epidemiology and Biostatistics.

Hannah Yang
Hannah P. Yang, Sc.M., joined HREB as a research fellow. For her doctoral dissertation at Johns Hopkins University, she is examining the effects of smoking and genetic variation in the estrogen-metabolizing genes on endometrial carcinogenesis. She is working with Montserrat García-Closas, M.D., Dr.P.H., on the Polish Breast, Ovarian, and Endometrial Cancer Study.
After more than three years as a visiting fellow in the Occupational and Environmental Epidemiology Branch, Honghong Zhu, M.D., Ph.D., successfully defended her doctoral dissertation at Johns Hopkins University and has left DCEG to pursue a new position.

Susan Devesa with Joseph Fraumeni
II December, Susan S. Devesa, Ph.D., retired from BB after more than 40 years of federal civil service. She received an M.H.S. and Ph.D. in epidemiology from the Johns Hopkins School of Hygiene and Public Health. She joined NCI in 1966 and was appointed Chief of the Descriptive Studies Section in 1993. She led the publication of the Atlas of Cancer Mortality in the United States, 1950–94, which was incorporated into the Cancer Mortality Maps and Graphs web site. She has coauthored more than 200 scientific articles and chapters, many of which focused on the temporal and demographic patterns in cancer rates according to histopathologic type and subsite to generate etiologic hypotheses, evaluate consistency with other hypotheses, and identify cancers warranting special study. She is a member of the American College of Epidemiology and has received the PHS Special Recognition Award for descriptive studies of the changing patterns of cancer in the United States, which have provided new clues to etiologic risk factors. She also received the DCEG Exemplary Service Award in 2004 in recognition of sustained research accomplishments and outstanding service to the Division and NCI. She continues to collaborate with DCEG researchers.

nCI amonG besT PlaCes To work For PosTDoCs
NCI, including DCEG, the Division of Cancer
Prevention, and the Center for Cancer
Research, ranked as one of the top 15 “Best Places
to Work” for postdoctoral fellows in 2007. Furthermore,
NCI is the only institute to be ranked in the top 15
for five consecutive years, based on annual surveys
of postdoctoral fellows around the world conducted
by The Scientist. According to satisfied fellows,
NCI follows a simple recipe of well-equipped research
facilities, helpful mentors and knowledgeable
colleagues, the freedom to explore new ideas,
and reasonable money and benefits.
Forum evaluaTes GeneTIC CounselInG anD TesTInG Issues
In 2005, the Institute of Medicine (IOM) of the National Academies established a National Cancer Policy Forum to allow government, industry, academic, consumer, and advocate representatives to discuss subjects relevant to preventing, curing, and palliating cancer. Both governmental and nongovernmental organizations sponsor the Forum and its activities, which include workshops, symposia, and reports to inform members and the IOM about critical cancer policy issues and to plan formal IOM studies of those issues. Dr. Hal Moses, Vanderbilt-Ingram Cancer Center, is the Forum’s chair; its 15 members include John E. Niederhuber, M.D., Director of NCI, and Joseph F. Fraumeni, Jr., M.D., Director of DCEG.
The Forum recently held a workshop on on “Cancer-related genetic counseling and testing” in Washington, DC, on March 30, 2007, to: (1) assess whether the United States has sufficient workforce resources to meet escalating needs for quality clinical cancer genetics services, (2) identify impediments to providing such services, and (3) initiate a process leading to recommendations regarding how these constraints might be eliminated. Participants included the members of the Forum, some of whom also served as presenters for specific topics, and a limited number of guest presenters. Mark H. Greene, M.D., Chief of the Clinical Genetics Branch, discussed “Health care provider supply and preparedness.” Using his experience as study chair of GOG-199, the National Ovarian Cancer Prevention and Early Detection Study, as a model, Dr. Greene described how genetic counselors, advanced practice oncology nurses, social workers, primary and specialty care physicians (including medical geneticists, medical oncologists, and gynecologists), and clinical investigators are essential to cancer risk assessment, counseling, and testing.
Recent projections show a serious shortage of medical oncologists (currently, a major source of cancer genetics care) over the next 15 years.
Dr. Greene focused on problems faced by these providers and, consequently, by those who need genetic counseling. These problems include serious deficiencies in acquiring and interpreting family history information by physician providers, the striking downward trend in board-certified medical geneticists graduating from genetics training programs, and a lack of awareness of genetic disorders and their evaluation among providers of health care to adults—partly a consequence of genetics’ historical origins as a discipline within pediatrics and obstetrics. Barriers to incorporating a genetic curriculum into medical school, residency and fellowship training programs, and certification examinations also exist. In addition, recent projections show a serious shortage of medical oncologists (currently, a major source of cancer genetics care) over the next 15 years.
Dr. Greene also observed that advanced practice oncology nurses are underused and that, surprisingly, the number of trained genetic counselors is growing more slowly than expected. Current membership in the National Society of Genetic Counselors comprises 1,912 trained genetic counselors, with only 14 percent spending more than half their time on cancer genetics. Organizations such as the American Academy of Family Physicians and the American Society of Clinical Oncology have made special efforts to proactively train and educate their membership about the newly evolving principles of cancer genetics.
Other topics presented at the Forum included: promises and pitfalls of cancer-related genetic counseling and testing, current and future demand for cancer-related genetic testing and counseling services, implications of home tests and direct-to-consumer advertising, providing community-based services, reaching underserved populations, reimbursement issues, psychological impact on patients and families, and implications for health and life insurance.
—Mark H. Greene, M.D.