IN THIS ISSUE:


| Other Issues | ||
|---|---|---|
| Mar 2006 PDF format |
 |
|
| Archived Issues | ||
Methods to Minimize False Positive Findings
Thanks to leaps in technology, potentially exciting findings in the molecular epidemiology of cancer are available faster than ever before. As with all areas of progress, there are challenges.
DCEG Director Joseph F. Fraumeni, Jr., M.D., said, “The emergence of new genomic technologies has provided epidemiologists with powerful tools to identify genetic variants that predispose to common diseases, including cancer. But progress has been slowed by a disconcerting number of inconsistent associations reported in the literature.”
The problems, he says, are being solved in two ways:
- New statistical approaches, such as the false positive report probability (FPRP) statistic.
- Collaborative epidemiologic strategies including cohort, case-control, and family-based consortia that enable pooling of large datasets for statistical power and the opportunity to replicate positive findings rapidly and efficiently.
 A
2004 paper in the Journal of the National Cancer Institute, by Sholom
Wacholder, Ph.D., a senior investigator in the Biostatistics Branch,
and others, introduced the FPRP statistic as a way to limit the number
of false positive results. The FPRP statistic can be calculated using the
observed p value, the “prior probability” of an association
as estimated by the investigator, and the study’s power to detect
the association.
A
2004 paper in the Journal of the National Cancer Institute, by Sholom
Wacholder, Ph.D., a senior investigator in the Biostatistics Branch,
and others, introduced the FPRP statistic as a way to limit the number
of false positive results. The FPRP statistic can be calculated using the
observed p value, the “prior probability” of an association
as estimated by the investigator, and the study’s power to detect
the association.
“In a statistical test, you’re making a decision, saying ‘This test result is significant and worthy of note’ or ‘We do not see compelling evidence of an association,’” Dr. Wacholder said. “There are too many declarations of significance under the usual test of significance, a p value of 0.05, when you have a prior probability of something like 1 in 1,000.”
The FPRP statistic, Dr. Wacholder said, “allows each hypothesis to be evaluated on its own, and it takes into account not only the data but also other information available about the hypothesis. I think this is the way scientists work: They temper their enthusiasm for surprising findings by considerations of plausibility.”
Montserrat Garcia-Closas, M.D., Ph.D., a tenure-track investigator in the Hormonal and Reproductive Epidemiology Branch, was a coauthor on the FPRP manuscript. “FPRP is a useful tool to evaluate how robust findings are, taking into account our prior knowledge” she said.
Dr. Garcia-Closas, who attended medical school in her native Spain and received a doctoral degree in epidemiology from the Harvard School of Public Health, is also a leader in conducting large-scale collaborative studies that are designed to avoid some of the pitfalls of false positive findings. Her work is focusing on genetic susceptibility to breast and bladder cancers.
Her breast cancer research has involved two large population-based studies in the United States and Poland. Together, these case-control studies involved more than 5,300 cases and 5,200 controls. Three papers in press show that missense mutations in the ATM gene are likely to influence breast cancer risk, whereas the evidence was weaker or not present for genetic polymorphisms in other double-strand break repair genes or base-excision repair genes. “We are able to look across two studies to see if a finding in one study is replicated in the other,” Dr. Garcia-Closas said. She is also involved with two consortia of breast cancer studies involving other case-control studies as well as cohort studies that are generating data on several thousand cases.
Dr. Garcia-Closas is also incorporating in her epidemiologic studies both traditional and molecular pathology approaches in a search for heterogeneity in breast cancer. Early results from the Polish study suggest that different tumor types—as characterized by histology, grade, size, and nodal status—might actually be etiologically distinct. She and colleagues are also using tissue microarrays containing tumor tissue from breast cancer patients in the Polish study to look at molecular markers in breast cancer. The first markers under study—the estrogen receptor, progesterone receptor, and HER2/neu—were quantitatively evaluated using an automated fluorescent staining technique, in addition to standard immunohistochemistry.
“In a statistical test, you’re making a decision, saying ‘This test result is significant and worthy of note’ or ‘We do not see compelling evidence of an association.…’ There are too many declarations of significance under the usual test of significance, a p value of 0.05, when you have a prior probability of something like 1 in 1,000.”
“Next we’ll move to molecular classification of tumors identified by gene expression microarrays,” Dr. Garcia-Closas said. “Later we’ll move to more exploratory classification—markers of hormone action, metabolism, and others—but we’re starting with factors that have well-described clinical relevance.”
Figure 1. Sample size needed to achieve a FPRP value of 0.1 with various prior probabilities or with an alpha level of 0.5 (black broken line) for traditional sample size calculations. Sample size is shown for various allele frequencies, with statistical power of 0.8 to detect an odds ratio of 1.5. (Wacholder S, et al. 2004) |
Dr. Garcia-Closas’s research on bladder cancer has focused on the risk related to variants in metabolic and DNA repair genes. A study recently published in Lancet provided compelling evidence that two genetic polymorphisms—NAT2 slow acetylation and GSTM1 null genotype—are associated with an increased risk of bladder cancer. Dr. Garcia-Closas and colleagues recently formed the International Consortium of Case-Control Studies of Bladder Cancer so that they can more easily conduct pooled and parallel analyses to confirm and clarify findings. This consortium approach, Dr. Fraumeni said, “provides an opportunity to combine datasets for large-scale studies that are needed to estimate the cancer risks associated with susceptibility genes and to conduct subset analyses that may help uncover gene-gene and gene-environment interactions.”
To Dr. Garcia-Closas, the future of molecular epidemiology has the potential to be very bright. “I think we now have the technology to explore common genetic variation, which could have a sizable impact on cancer,” she explained. “We used to look at a few genetic variants and now we can look at several thousand, in an affordable and much more efficient way.” In addition, she continued, “another limitation was the relatively small size of individual studies. Now the studies are larger and the consortia being formed are accelerating this trend.”
One exciting avenue for genetic susceptibility research is whole-genome scans, she said. “Right now we explore genetic susceptibility based on current knowledge on pathways to cancer, but we might be missing mechanisms that we don’t understand so well. The scans might let us identify novel genes and etiologic pathways.”
DCEG Linkage is a publication of the Division
of Cancer Epidemiology and Genetics, National
Cancer Institute.
Joseph F. Fraumeni, Jr., Director
Shelia Hoar Zahm, Deputy Director
Managing Editor
Samantha Nhan (nhans@mail.nih.gov)
Scientific Highlights Editor
Patricia Madigan (madiganp@mail.nih.gov)
DCEG Linkage Reporters
Office of the Director
Sandy Rothschild (rothscsa@mail.nih.gov)
Epidemiology and Biostatistics Program
Geoffrey Tobias (tobiasg@mail.nih.gov)
Biostatistics Branch
B.J. Stone (stoneb@mail.nih.gov)
Clinical Genetics Branch
June Peters (petersju@mail.nih.gov)
Genetic Epidemiology Branch
Mary Fraser (fraserm@mail.nih.gov)
Barbara Rogers (rogersb2@mail.nih.gov)
Hormonal and Reproductive Epidemiology Branch
Patricia Madigan (madiganp@mail.nih.gov)
Nutritional Epidemiology Branch
Amanda Cross (crossa@mail.nih.gov)
Occupational and Environmental Epidemiology Branch
Phyllis Nimeroff (pnimerof@mail.nih.gov)
Radiation Epidemiology Branch
Jenna Nober (noberj@mail.nih.gov)
Viral Epidemiology Branch
Julie Grey (jrussell@mail.nih.gov)
DCEG Committee of Scientists
Aaron Blair (blaira@mail.nih.gov)
DCEG Representative to the NIH Women Scientists
Advisory Group
Lynn Goldin (goldinl@mail.nih.gov)
DCEG Representative to the NIH Tenure-track
Investigators Committee
Alice Sigurdson (sigurdsa@mail.nih.gov)
DCEG Representatives to the NIH Fellows Committee
Shih-Chen Chang (changshi@mail.nih.gov)
Hong Hong Zhu (zhuh@mail.nih.gov)
Palladian Partners, Inc.
Robin Moore (rmoore@palladianpartners.com)
DCEG LINKAGE RECEIVES DISTINGUISHED AWARDLinkage received the highest category of recognition as a “Distinguished Technical Communication” in the 2005 competition sponsored by the Washington, DC, chapter of the Society for Technical Communication. In their overall evaluation, the judges cited the organization and visual appeal of Linkage, noting that it does an excellent job of presenting complex information in a way that is both understandable and interesting to a wide and diverse scientific and lay audience. The publication was judged to be superior in every respect, as well as an outstanding example of the application of technical communication principles. In his announcement, DCEG Director Joseph F. Fraumeni, Jr., M.D., acknowledged the hard work not only of the Office of the Director’s Samantha Nhan, Managing Editor of Linkage, but also the many other DCEG contributors, and he congratulated them for their outstanding efforts and “a job well done.” |
|
SOPHIA WANG: TENURE-TRACKER TACKLES TWO TUMORS
Sophia Wang, Ph.D., a tenure-track investigator in the Hormonal and Reproductive Epidemiology Branch (HREB), has developed a diverse portfolio in two distinct areas of epidemiologic research: non-Hodgkin lymphoma (NHL) and cervical cancer. “I strongly believe that my work in one research area also informs what I do in another, and makes me a better investigator,” Dr. Wang said.
When Dr. Wang joined the NCI in 2000, data collection for the large interdisciplinary NCI/SEER (Surveillance, Epidemiology, and End Results) case-control study of NHL had just been completed. Led by Patricia Hartge, Sc.D., Deputy Director of the Epidemiology and Biostatistics Program, the population-based study included 1,321 cases and 1,057 controls and was conducted in four different areas around the United States where SEER registries are located. Study participants were interviewed about a wide range of risk factors, and biospecimens were also collected.
“Dr. Hartge and the study group had created exactly the type of environment that most appealed to me,” Dr. Wang said. “The study serves so many people on so many levels—it’s truly a collaborative effort.” Dr. Wang has been deeply involved in the study since her arrival, overseeing specimen handling, genotyping, and biological specimen analysis.
 Dr.
Wang recently coauthored a pooled analysis of 12 single nucleotide polymorphisms
that linked certain inflammatory response genes to increased risk of
NHL. The study, which was published in the January issue of Lancet
Oncology, found an increased risk of diffuse large B-cell lymphoma
in individuals who were homozygous for the TNF-308A allele and who carried
at least one IL10-3575A allele. Data from 3,586 cases and 4,018 controls
were pooled from NHL investigators participating in InterLymph—the
International Lymphoma Epidemiology Consortium—from a dozen countries
around the world.
Dr.
Wang recently coauthored a pooled analysis of 12 single nucleotide polymorphisms
that linked certain inflammatory response genes to increased risk of
NHL. The study, which was published in the January issue of Lancet
Oncology, found an increased risk of diffuse large B-cell lymphoma
in individuals who were homozygous for the TNF-308A allele and who carried
at least one IL10-3575A allele. Data from 3,586 cases and 4,018 controls
were pooled from NHL investigators participating in InterLymph—the
International Lymphoma Epidemiology Consortium—from a dozen countries
around the world.
Members of InterLymph initiate collaborative projects and merge data across studies to test specific hypotheses. Dr. Wang has been involved with InterLymph since its inception and gives it rave reviews. “It’s a friendly and nurturing environment for research,” she said. “We’ve been able to conduct parallel and pooled analyses with great success—all the investigators have been very cooperative and helpful.”
In addition to Dr. Hartge, Dr. Wang credits Nathaniel Rothman, M.D., M.P.H., M.H.S., Occupational and Environmental Epidemiology Branch, and Martha Linet, M.D., M.P.H., Chief of the Radiation Epidemiology Branch, with spearheading the InterLymph effort at NCI. Her current InterLymph collaboration involves conducting a pooled analysis of family history of cancer and NHL risk.
Dr. Wang’s second major research area involves the natural history studies of cervical cancer. She is leading SUCCEED (Study to Understand Cervical Cancer Early Endpoints and Determinants), where she is examining intermediate stages of cervical carcinogenesis to understand disease progression.
“One of the main reasons I joined DCEG was that I wanted the opportunity to be an independent investigator and conduct research that I cared deeply about,” she said. “Louise Brinton, Ph.D., (Chief of HREB) has been wonderful in nurturing tenure-track investigators such as myself and supporting us as independent investigators. It is easy to be inspired by the legacy of HREB cervical cancer studies conducted by Dr. Brinton, Mark Schiffman, M.D., M.P.H., and Allan Hildesheim, Ph.D. Cervical cancer is an ideal model tumor to study; with precursor lesions and the etiologic agent known, it provides a unique opportunity to use cutting-edge technology to truly understand the gene expression profiles at each stage of disease progression. By doing this, we can identify biomarkers of early detection for cervical cancer and help inform carcinogenesis in general.”
Dr. Wang studied molecular biology at the Massachusetts Institute of Technology, where her senior thesis advisor thought an introduction to David Hunter, Sc.D., of the Harvard School of Public Health might prove beneficial.
“Hearing Dr. Hunter describe the goals of the Channing Laboratory, I realized that I could take my skills in molecular biology and apply them to studies of human populations,” Dr. Wang recalled. She earned her Ph.D. in epidemiology from Johns Hopkins University with support from an NCI cancer epidemiology training grant.
Dr. Wang then completed two years as an Epidemic Intelligence Service Officer at the Centers for Disease Control and Prevention. “My mentor, Muin Khoury, M.D., was so inspiring that he sealed my interest in genetic epidemiology,” she said. “He also expanded the breadth of my experience, enabling me to investigate the avian influenza outbreak in Hong Kong that provided invaluable experience as a shoe-leather epidemiologist. As it turns out, having a good working knowledge of viral genetics has proven particularly useful, since I am drawn to tumors with known or suspected infectious etiologies.” (Human papillomavirus is the central etiologic agent of cervical cancer, and NHL has been linked with Epstein-Barr virus, hepatitis C virus, and HIV.)
“InterLymph … [is] a friendly and nurturing environment for research.… We’ve been able to conduct parallel and pooled analyses with great success.”
Not only is Dr. Wang involved in two areas of cancer research, she is also passing along her knowledge and skills to others, which she sees as the key to scientific progress.
“I think one of the things I’m most proud of is that by becoming well-versed in specific areas, I have the opportunity to convey that knowledge to others,” she said. “Working with talented students and postdoctoral fellows is a real treat, and mentoring them so that they can pursue good ideas and become independent investigators is particularly satisfying.”
NIH RECOGNIZES NINE FARE WINNERS FOR 2006The NIH Fellows Award for Research Excellence (FARE) program recognizes outstanding scientific research by intramural postdoctoral fellows. In 2006, nine DCEG fellows were award recipients. To enter the competition, fellows submit abstracts of their research, which are reviewed by a panel of NIH postdoctoral fellows and tenured/tenure-track investigators. Winners receive a travel stipend to attend a scientific meeting, where they present their research papers. More information about the FARE competition is available at http://felcom.nih.gov/FARE. DCEG FARE Winners and Abstract Titles Biostatistics Branch
Clinical Genetics Branch
Genetic Epidemiology Branch
Hormonal and Reproductive Epidemiology Branch
Nutritional Epidemiology Branch
Occupational and Environmental Epidemiology Branch
|
PAOLO VINEIS VISITS DCEG AS DISTINGUISHED LECTURER
Paolo Vineis, M.D., M.P.H., visited NCI from November 3–4, 2005, as an invited lecturer for the DCEG Distinguished Lectures in Occupational and Environmental Cancer. Dr. Vineis is the Chair in Environmental Epidemiology in the Division of Epidemiology, Public Health, and Primary Care in the School of Medicine at Imperial College in London. A leader in the field of molecular epidemiology, Dr. Vineis conducts research focusing on cancer and genetic susceptibility in relation to DNA repair and the metabolism of carcinogenic substances. Dr. Vineis is also one of the key coordinators for the European Prospective Investigation and Cancer project, a large cohort study of diet and cancer. He has served numerous times on expert working groups and advisory boards.
Dr. Vineis’s lecture on “The integration of mechanistic data into the evaluation of environmental carcinogens” underscored the importance of molecular epidemiology for research on cancer etiology. He illustrated his theme with several examples of the use of biomarkers for assessing carcinogenic effects, including those resulting from exposure to environmental tobacco smoke, styrene, and ethylene oxide. He also discussed the challenges of studying gene-environment interactions and expressed concern that current measures for exposure assessment, even including biomarkers of exposure, have not yet reached the degree of sophistication of modern genetic analysis. This could lead to a greater degree of classification error for these markers and increased potential for false negative findings. For genetic studies, assay reliability is generally not a problem, but newer high-throughput methods threaten to produce large numbers of false positive findings.

Dr. Vineis highlighted several approaches to improve exposure assessment in epidemiologic studies. One method is to use repeated exposure measures to allow for regression dilution bias. He also called for careful validation of novel analytic methods to detect proteomic and metabolomic signatures in body fluids of metabolic processes and external exposures. He concluded his talk by considering how mechanistic data have been successfully included in the evaluation process for identification and risk assessment related to environmental carcinogens.
Following Dr. Vineis’s talk, Dalsu Baris, M.D., Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), coordinator of the Distinguished Lecturer Series, presented Dr. Vineis with a plaque in recognition of his valuable contributions to the fields of occupational and environmental epidemiology.
Dr. Vineis’s two-day visit also involved a second seminar for OEEB staff entitled “The GENAIR study: Cancer, air pollution, and environmental tobacco smoke” and meetings with DCEG investigators and fellows.
NCI AMONG TOP 15 BEST PLACES TO WORKThe NCI intramural training programs, including the Center for Cancer Research, the Division of Cancer Epidemiology and Genetics, and the Division of Cancer Prevention, were voted one of the “Top Fifteen Best Places to Work for Postdoctoral Scientists” in 2005. NCI moved up to third place in the rankings, from fourth place in 2004. The ranking is based on an annual survey of the scientific community in the United States and Canada conducted by The Scientist. This institutional kudo continues the streak of consistently high ratings that the fellowship programs across NCI have received over the past several years. |
|
BREAST CANCER RISK IN ATAXIA-TELANGIECTASIA FAMILIES
Margaret Tucker, M.D., Chief of the Genetic Epidemiology Branch, and Ruth Kleinerman, M.P.H., Radiation Epidemiology Branch, organized an international workshop entitled “Population-based studies of breast cancer risk in relatives of ataxia-telangiectasia patients.” The workshop, sponsored by the Office of Rare Diseases, was held on September 26, 2005, at the headquarters of the Danish Cancer Society in Copenhagen. Jorgen Olsen, M.D., Director of the Institute for Epidemiologic Research at the Danish Cancer Society, cohosted the workshop.
The goals of the workshop were to explore the development of collaboration between investigators with published population-based studies of cancer risk in families with the ataxia-telangiectasia mutated gene (known as ATM), to identify specific scientific questions that could be answered by data from these studies, and to define a common approach to epidemiologic and mutation data analyses. Most attendees came from France, the United Kingdom, and the Nordic countries. They were charged with defining the opportunities to determine whether breast cancer risk is causally related to the ATM gene and what resources will be needed for a productive collaboration.
The first half of the meeting was dedicated to the presentation of epidemiologic and mutation data from existing studies conducted by the French, U.K., and Nordic investigators. These studies have accrued data for more than 4,000 relatives in 232 families with an ataxia-telangiectasia child. The second half of the meeting was spent identifying research questions that could be answered by pooled analyses and developing criteria for pooling. The possibility of adding population-based studies from other countries was discussed. Lead roles for various study tasks (protocol development, analytic strategies, mutation analysis) were determined, and three papers were planned.
The group made tentative plans to meet again in June 2006 to review preliminary pooled analyses.
NIH-AARP FELLOW JOINS THE DIVISIONYikyung Park, Sc.D., recently joined the Nutritional Epidemiology Branch (NEB) as the first NIH-AARP fellow, a position sponsored in part by the AARP. Dr. Park earned a B.S. in food and nutritional science and a M.S. in nutritional biochemistry from Ewha Womans University in Seoul, South Korea. She continued her studies in nutritional epidemiology at the Harvard School of Public Health, where she received a doctorate. Her dissertation, which analyzed data pooled from 13 prospective cohort studies, focused on the association between dietary fiber and colorectal cancer risk and was recently published in the Journal of the American Medical Association. “I wanted to come to the National Cancer Institute as a postdoctoral fellow,” Dr. Park said, “because it is such a great environment for cancer research, and for the wonderful opportunity it would provide me to try new things.” In DCEG, Dr. Park’s research projects include the NIH-AARP Diet and Health Study, a large prospective cohort study of diet and cancer among more than 500,000 AARP members between the ages of 50 and 71. Under the mentorship of Arthur Schatzkin, M.D., Dr.P.H., Chief of NEB, Dr. Park hopes to examine a variety of nutritional factors within this study, particularly the consumption of fruits and vegetables, calcium intake, and their effects on the risk of different types of cancer. “This new fellowship represents a great opportunity for us and a new development in our ongoing collaboration with AARP,” Dr. Schatzkin said. “Dr. Park is extremely talented and well-trained in the analysis of cohort data. We are excited to have her join NEB.” |
|
CANCER RISKS FROM NUCLEAR WEAPONS TESTING FALLOUT
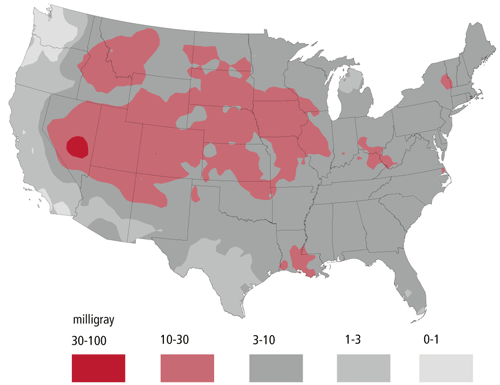
Fifty years ago, the United States tested nuclear weapons in the Nevada desert. Radioactive debris from the tests became airborne and was deposited downwind as “fallout,” predominantly in states east and north of the test site. Residents of these areas were exposed to radiation from the fallout, in particular to radioactive iodine (I-131) that found its way into milk and dairy products produced by cows, sheep, and goats that grazed on contaminated vegetation. Some contaminated dairy products also entered the national food supply, so people in other areas of the United States were also exposed to I-131. Individuals who consumed these milk and dairy products as children are now at higher risk for developing thyroid disease and abnormalities, including thyroid cancer. Exposure to gamma radiation from the fallout has also resulted in an increased risk of leukemia, though the risk is much less than that for thyroid cancer.
The publication highlights an interactive web-based calculator, developed by REB investigators, that permits users to estimate their thyroid radiation dose from Nevada fallout and the related risk of developing thyroid cancer.
An overview of radioactive fallout from nuclear weapons testing and related cancer risks was recently published by Steven Simon, Ph.D., Andre Bouville, Ph.D., and Charles Land, Ph.D., of the Radiation Epidemiology Branch (REB) in the American Scientist. The paper describes the history of atmospheric nuclear testing, explains how radioactivity in fallout is transferred through the environment and results in human exposure, discusses the meaning of radiation dose, and provides estimates of dose and related cancer risks to the American people.
|
The article summarizes the findings of several REB projects on fallout exposures from nuclear tests carried out in Nevada and elsewhere in the world. It reconstructs the likely radiation doses to affected populations and estimates their cancer risk from this exposure. The publication highlights an interactive web-based calculator, developed by REB investigators, that permits users to estimate their thyroid radiation dose from Nevada fallout and the related risk of developing thyroid cancer. Available online, the I-131 Thyroid Dose/Risk Calculator estimates exposure to I-131 based on where users lived from 1951 to 1971 and how much milk they may have consumed. The calculator is easy to use and is the only tool of its kind available for public use. The dose/risk calculator is available at http://ntsi131.nci.nih.gov.
Much of the research on I-131 conducted in the REB has been in response to congressional requests that were fueled by public concern about radiation risks and the growing knowledge of the long-term health consequences of nuclear testing conducted during the 1950’s and 1960’s. The article also explains how the knowledge gained from years of studying radioactive fallout and nuclear accidents can provide an understanding and basis for preparing for terrorist attacks that might involve radioactive materials.
NIH RESEARCH FESTIVAL
The 18th annual NIH Research Festival was held October 18–21, 2005. This two-and-a-half day event showcases the achievements of intramural investigators and provides an opportunity to explore the breadth and depth of research conducted across the NIH Intramural Research Program.
This year, DCEG investigators Patricia Hartge, Sc.D., Deputy Director of the Epidemiology and Biostatistics Program, Stephen Chanock, M.D., Director of the Core Genotyping Facility, Nathaniel Rothman, M.D., M.P.H., M.H.S., a senior investigator in the Occupational and Environmental Epidemiology Branch, and Sophia Wang, Ph.D., an investigator in the Hormonal and Reproductive Epidemiology Branch, along with Sandeep Dave, M.D. (NHLBI), and Michael Lenardo, M.D. (NIAID), led a panel on the “Molecular epidemiology of non-Hodgkin lymphoma (NHL): Etiology, prognosis, and underlying mechanisms.” The discussion focused on the need to identify factors that play a role in preventive and therapeutic strategies. DCEG research has demonstrated that environmental and host factors are implicated in the etiology of NHL, a disease for which incidence rates have steadily increased since 1950.

Andrew Bergen, Ph.D., Genetic Epidemiology Branch, Michael Leitzmann, M.D., Dr.P.H., Nutritional Epidemiology Branch (NEB), Panagiota Mitrou, Ph.D. (NEB), and Preetha Rajaraman, Ph.D., Radiation Epidemiology Branch, presented posters that described their research. Kristin Kiser, M.H.A., Program Coordinator in the Office of Education, and Sandy Rothschild, Office of the Director, manned the Division’s exhibit booth and provided materials on DCEG research highlights, copies of Linkage newsletters, and educational information.
FELLOW COAUTHORS GENETICS CURRICULUMChristine Mueller, D.O., Clinical Genetics Branch (CGB), has coauthored a clinical genetics curriculum, released in October 2005, for the American Academy of Family Practice (AAFP). This home-study, self-assessment program is intended to educate family practitioners in key principles of applied and theoretical genetics. It is designed to “provide clinicians with basic tools for reading and understanding the genetic literature, an overview of some of the more frequently encountered issues related to genetics in family medicine, and a glimpse into future developments in genetics.” It is being widely distributed nationally by the AAFP as part of their comprehensive Continuing Medical Education program. This project complements similar work by other CGB staff in developing educational materials for medical oncologists, oncology nurses, genetic counselors, marriage and family therapists, social workers, and high-risk patients. These efforts contribute to CGB’s goal of advancing knowledge about genetics in both professional and lay communities, and translating modern molecular biology into evidence-based improvements in patient care. |
|
DCEG WELCOMES VISITING SCHOLAR MUIN KHOURY
 DCEG
hosted Muin Khoury, M.D., as its most recent Visiting Scholar on September
15–16, 2005. A leader in the field of genetic epidemiology, Dr.
Khoury is the founding Director of CDC’s Office of Genomics and
Disease Prevention, which assesses the impact of advances in human genetics
and the Human Genome Project on public health and disease prevention
and integrates genomics into public health research. Dr. Khoury, the
author of the classic textbook Fundamentals of Genetic Epidemiology, lectures
extensively and holds faculty positions at the Emory Rollins and Johns
Hopkins Bloomberg Schools of Public Health. A leading figure in the study
of interactions between genes and environment, he has chaired several
national task forces and given congressional testimony on the importance
of these interactions to public health. Dr. Khoury has received numerous
awards in recognition of his scholarship and leadership, including the
Public Health Service Special Recognition Award, the Arthur Fleming Award,
and the CDC Research Honor Award.
DCEG
hosted Muin Khoury, M.D., as its most recent Visiting Scholar on September
15–16, 2005. A leader in the field of genetic epidemiology, Dr.
Khoury is the founding Director of CDC’s Office of Genomics and
Disease Prevention, which assesses the impact of advances in human genetics
and the Human Genome Project on public health and disease prevention
and integrates genomics into public health research. Dr. Khoury, the
author of the classic textbook Fundamentals of Genetic Epidemiology, lectures
extensively and holds faculty positions at the Emory Rollins and Johns
Hopkins Bloomberg Schools of Public Health. A leading figure in the study
of interactions between genes and environment, he has chaired several
national task forces and given congressional testimony on the importance
of these interactions to public health. Dr. Khoury has received numerous
awards in recognition of his scholarship and leadership, including the
Public Health Service Special Recognition Award, the Arthur Fleming Award,
and the CDC Research Honor Award.
In his keynote address, “Public health genomics: Using genetic knowledge for public health,” Dr. Khoury noted the acceleration of technology in genetics and challenged the cancer epidemiology community to change in fundamental ways. He argued for new models of data sharing, analysis, and rapid public dissemination of results, and he offered HuGENet (Human Genome Epidemiology Network) as one model. Dr. Khoury initiated HuGENet several years ago in an effort to encourage data pooling as a means to derive more accurate estimates of specific genetic effects on disease risk. Citing the immense influence of genomic factors on population health as an overarching theme, he touched briefly on the public health impact of genetic tests for screening and prevention and the use of family history as a tool for disease prevention and public health. At the conclusion of Dr. Khoury’s presentation, Demetrius Albanes, M.D., Chief of the Office of Education and Senior Investigator in the Nutritional Epidemiology Branch, presented him with a plaque in recognition of his outstanding contributions to the field of genetic epidemiology.
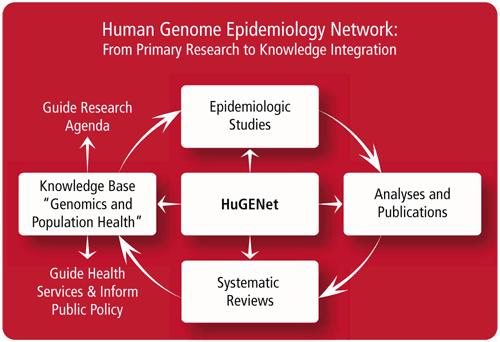
The remainder of the two-day visit was devoted to a series of stimulating group discussion sessions and meetings with individuals and branches. In a group discussion on epidemiological networks and consortia moderated by Thomas O’Brien, M.D., M.P.H., Hormonal and Reproductive Epidemiology Branch (HREB), and Patricia Hartge, Sc.D., Deputy Director of the Epidemiology and Biostatistics Program, DCEG investigators reported on more than a dozen cancer epidemiology consortia now actively working to conduct pooled and parallel analyses and to foster collaborations. Dr. Khoury described his newest venture, “A Network of Networks,” a community of scientists involved in research consortia, of which DCEG is a member.
Phillip Rosenberg, Ph.D., Biostatistics Branch (BB), hosted a journal club discussion of Dr. Khoury’s recent article, “Do we need genomic research for the prevention of common diseases with environmental causes?” A related group discussion on statistical approaches to the investigation of risk factor combinations followed, led by Sholom Wacholder, Ph.D. (BB), and Montserrat Garcia-Closas, M.D., Ph.D. (HREB). During these sessions, Dr. Khoury and DCEG scientists probed the statistical, organizational, and cultural challenges involved in translating findings from genetic epidemiological studies into public health actions.
Dr. Khoury discussed current issues in studying cancer-prone families and in patient counseling with members of the Genetic Epidemiology Branch (GEB) and the Clinical Genetics Branch. Margaret Tucker, M.D., Chief of GEB and Director of the Human Genetics Program, moderated a group session that focused on current challenges in genetic epidemiology. According to Dr. Khoury, these challenges include scientific issues such as design, analysis, and the juxtaposition of family and population studies, and logistical considerations such as ethical review of human subjects research and HIPAA regulations; response rates; the size and cost of repositories; and budgeting for high-throughput genotyping, expression arrays, and proteomics during austerity periods.
Dr. Khoury also met with scientists in the Health Communications Branch of the Division of Cancer Control and Population Sciences (DCCPS) and with DCCPS Director Robert Croyle, Ph.D. His visit concluded with a roundtable discussion where he met with postdoctoral fellows and offered his suggestions for scientific career development.
NCI DIRECTOR’S AWARDSCongratulations to the following DCEG staff members, who were recognized for their accomplishments over the past year at the annual NIH Awards Ceremony, held on October 26, 2005. The following staff received NIH Merit Awards from NCI Director Andrew C. von Eschenbach, M.D.:
Finally, Sharon Miller of the NCI Research Contracts Branch received the NCI Director’s Gold Star Award in recognition of a special accomplishment that advances the NCI agenda in a significant and meaningful way.
|
 |
NEW NCI DIRECTOR’S INNOVATION AWARDS
The newly created NCI Director’s Innovation Award Program is designed to support the development of novel approaches and cutting-edge technology targeting high-impact, high-risk areas of cancer research. The Program offers two levels of awards: those for tenure-track or recently tenured principal investigators, who may apply for up to $50,000 of single-year funding, and Career Development Awards for postdoctoral fellows, staff scientists, staff clinicians, and senior scientists, which include a one-time award of up to $10,000.

John Niederhuber, M.D., NCI Deputy Director and Deputy Director for Translational and Clinical Sciences, announced the winners of these prestigious awards at a ceremony held during the NCI Intramural Scientific Retreat on January 11, 2006. The DCEG principal investigator award recipients were Christian Abnet, Ph.D., M.P.H., Nutritional Epidemiology Branch (NEB), for his proposal on “Automated measurement of tissue element concentrations by x-ray fluorescence spectroscopy for epidemiological studies”; Maria Teresa Landi, M.D., Ph.D., Genetic Epidemiology Branch (GEB), for her proposal that “MicroRNAs provide a functional link from molecular epidemiology to cancer progression”; and Jorge Toro, M.D. (GEB), for his proposal on “Identification and validation of novel protein partners of the fumarate hydratase protein.”
Career Development Award recipients were D. Michal Freedman, Ph.D., Radiation Epidemiology Branch, and Unhee Lim, Ph.D. (NEB), for their proposal on “Serum vitamin D and risk of lymphoid cancers in a prospective study”; Ola Landgren, M.D., Ph.D. (GEB), for his proposal on “Serum protein markers of early oncogenic events: A pre-diagnostic multiple myeloma study”; and Jim Vaught, Ph.D., Office of the Director, and Mark Cosentino, Ph.D., SAIC-Frederick, for their proposal on “Designing a novel cryococktail for peripheral blood mononuclear cell storage.”
IHOR MASNYK RECOGNIZED BY UKRAINIAN MINISTER OF FOREIGN AFFAIRSOn September 19, 2005, Ukrainian Minister of Foreign Affairs Borys Tarasiuk, Ph.D., visited the United States to meet with leading government officials and representatives of several Ukrainian organizations. During the meeting with Ukrainian groups, Dr. Tarasiuk spoke about the important role the Ukrainian diaspora has played in securing a future for the population of Ukraine. Many members of the Ukrainian community in the United States helped ensure the recent Ukrainian political struggle resulted in democratic reform. Dr. Tarasiuk awarded an honorable citation of the Ministry of Foreign Affairs to Ihor Masnyk, Ph.D., of the Radiation Epidemiology Branch, in recognition of his many years managing the collaborative research on the ongoing health issues related to the 1986 Chornobyl disaster. This research, conducted for NCI and other organizations, has been of immense value to Ukrainians both in Ukraine and around the globe. Dr. Masnyk has also participated in charitable work with U.S.-Ukrainian organizations. “This citation,” Dr. Tarasiuk said, “is a token of gratitude to Dr. Masnyk for his long-lasting contribution to the health of Ukrainian children, who suffered from the accident at the Chornobyl nuclear power station.” |
NEW FELLOWSHIP IN CANCER HEALTH DISPARITIES
In response to the need for cancer research directed toward elucidating the causes of cancer health disparities across populations, DCEG is teaming up with two other components of NCI, the Center to Reduce Cancer Health Disparities and the Division of Cancer Control and Population Sciences, to sponsor a new training program called the NCI Cancer Health Disparities Fellowship. Together, these groups have the scientific expertise, resources, and large population databases to investigate the demographic patterns of cancer and the determinants of cancer health disparities. Research will focus on identifying, explaining, and reducing the elevated rates of cancer among racial, ethnic, gender, socioeconomic, and geographic/regional subgroups of the U.S. population. These disparities in cancer incidence and mortality provide important clues to factors that affect cancer risk and prognosis in the general population as well as in population subgroups.
Like current postdoctoral fellows, cancer health disparities fellows will have a mentored research experience lasting up to five years. The fellows may work on descriptive, etiologic, or prevention projects, as well as issues related to cancer care policy and delivery. This type of research aims to bridge the gap between discovery and delivery by informing and developing translational research interventions that will help reduce cancer health disparities. The fellows will have access to a growing portfolio of studies and resources through which cancer health disparities can be studied.
Applicants should have an M.D., Ph.D., or other equivalent degree in epidemiology, biostatistics, genetics, or a related field; be pursuing a degree in these areas; or have a doctoral degree in the social and behavioral sciences or a related health research field. Applications will be accepted in spring 2006, with new fellows arriving in the summer and fall of 2006. For more information, see www.dceg.cancer.gov.
COMMISSIONED CORPS JUNIOR OFFICER ADVISORY GROUPClaudine Samanic, M.S.P.H., of the Occupational and Environmental Epidemiology Branch, has been elected chair of the Commissioned Corps Junior Officer Advisory Group (JOAG) of the U.S. Public Health Service. JOAG’s mission is to provide advice and consultation to the leadership of the Commissioned Corps, including the U.S. Surgeon General, on issues relating to professional practice and personnel activities affecting the Corps’ junior officers. In 1999, the Corps recognized the critical importance of obtaining
advice and consultation from its junior officers in formulating
policy and career-related programs. A small group was charged with
assessing the needs of junior officers; developing an advisory
panel to address those needs; and establishing committees to promote
recruitment, leadership, and professional development. In December
2001, the Surgeon General officially chartered JOAG, which is governed
by 20 voting members and represents every operating division within
the U.S. Department of Health and Human Services (DHHS). Lieutenant
Commander Samanic represents junior officers in the Commissioned
Corps’
transformation initiatives and serves as JOAG’s liaison to
the Surgeon General’s Professional Advisory Committee. In
September, she met with John Agwunobi, M.D., M.B.A., then nominee
for DHHS Assistant Secretary for Health, to discuss issues of concern
to junior officers in the Commissioned Corps.
|
COMMITTEE REPRESENTS DCEG SCIENTISTS
Aaron Blair, Ph.D., of the Occupational and Environmental Epidemiology Branch, has assumed a new role as Chair of the DCEG Committee of Scientists (COS), as Mary Lou McMaster, M.D., Genetic Epidemiology Branch, steps down at the end of her two-and-a-half-year term as COS Chair.
Background, structure, and representation
When DCEG was first established as a separate Division within the NCI Intramural Research Program, Division Director Joseph F. Fraumeni, Jr., M.D., saw the need to develop a new mechanism that would keep him attuned to the issues, challenges, and concerns facing DCEG scientific staff. The COS was created to serve this need and was given the task of making recommendations to facilitate communication across the Division at all scientific levels. The charge to the Committee was broad: In addition to fostering Division-wide communication, COS was asked to consider ways to improve administrative and research support that would promote successful, productive research. The Committee was also asked to identify approaches that would enhance morale and strengthen the scientific environment for DCEG scientists as well as promote opportunities for career development.
“The committee has been brilliant at identifying problems at the earliest stage, thus making it possible to take corrective steps that enrich the work life in the Division and empower staff to maximize their scientific potential.”
Today, COS plays an active role within the Division, with representation from all Branches and all levels of scientific staff. The COS Chair maintains office hours (from 10:00 a.m. to 12:00 p.m. on the last Friday of each month) to consult with any member of DCEG and also serves as a voting member of the DCEG Senior Advisory Group (SAG). All COS representatives are available to discuss potential issues and concerns with members of their respective Branches and professional categories, and these discussions frequently generate action items that are taken up at the monthly COS meetings and, when appropriate, by the SAG.
Over the course of its 10-year history, COS has spearheaded a variety of ways to enhance communications and interventions across the Division, including separate town meetings that the Division Director conducts with principal investigators, postdoctoral fellows, or staff scientists. COS was also instrumental in developing the Annual Survey of Branch and Division Management and creating annual awards that recognize outstanding publications by postdoctoral fellows and staff scientists. COS suggestions have led to changes in the annual personnel review of staff scientists, improvements to the process of manuscript review, better contract management, and access to various training resources.
Procedures
Issues or concerns can be brought to the attention of COS through a variety of channels, including direct communications between scientific staff and their COS representative or the COS chair, responses to the DCEG Annual Survey of Branch and Division Management, and matters that arise during the town meetings. The Committee evaluates all concerns and advises the Division Director on structural or procedural solutions. Periodically, the Committee invites the Division Director or Deputy Director to attend its meetings and present the status of previous COS recommendations and to hear about current activities and concerns that have newly arisen. COS also helps to play a role in resolving individual problems through discussion or by directing individuals to the appropriate services and channels at NIH. At the same time, COS members appreciate the need to protect the confidentiality of DCEG staff and take great care to preserve the confidentiality of scientists who bring issues forward for their consideration.
Outlook
|
MARGARET TUCKER APPOINTED HUMAN GENETICS PROGRAM DIRECTOR
Dr. Tucker’s commitment to the NCI and the Division began early in her career. While at Harvard Medical School, she worked with Fred Li, M.D., on a project investigating familial childhood cancer and volunteered in the NCI epidemiology program. These experiences piqued Dr. Tucker’s interest, and thus began her life-long fascination with the field of cancer genetics and epidemiology. “It was like being a detective,” she said, “and it was obvious that it was much more efficient to prevent cancer than to treat it once established.” Since that time, Dr. Tucker has played an important role in leading the Division’s efforts to discover and understand genetic links to cancer. After completing her internal medicine and oncology training at Stanford University, she returned to NCI under the mentorship of DCEG Director Joseph F. Fraumeni, Jr., M.D. She quickly became involved in studies of hereditary melanoma and familial lymphoma, both of which continue to this day. She also carried out a study of second malignancies after childhood cancer, the first-ever case-control study of ocular melanoma, and a large case-control study of cutaneous melanoma. Dr. Tucker became the Chief of the Family Studies Section in 1987, and as the group flourished, she was appointed founding Chief of GEB in 1992. While continuing to serve as Chief for GEB, Dr. Tucker in her new role will work with the Division Director in managing and strengthening DCEG’s intramural and collaborative research programs in cancer genetics. Her vision of the Program’s mandate includes continuing development of a comprehensive program of gene discovery, including high- and low-penetrant genes, quantification of disease risk related to genetic variation, design of new methodologies, detection of genetic and environmental modifiers of risk, and development of evidence-based clinical care and interventions for genetically prone individuals. This will involve continued work on the familial cancers that have long been the focus of substantial effort, including melanoma, chronic lymphocytic leukemia, Hodgkin and non-Hodgkin lymphoma, Waldenström’s macroglobulinemia, neurofibromatosis type 2, chordoma, breast and ovarian cancers, and testicular cancer as well as population studies of various cancers to identify susceptibility genes and gene-environment interactions. Although some genes have already been identified for several of these cancers, there are still others that remain to be discovered. “We are starting to find linkage regions of interest for certain cancers, such as lymphoproliferative tumors, and we will intensify work in these areas,” Dr. Tucker stated. “But also we have to keep in mind that finding high-risk susceptibility genes is only the start. There are other genes and interactions that are important to understanding the etiology of cancers that are likely to affect the general population. Our interdisciplinary approach will continue to focus not only on family studies, but also large-scale population studies and clinical trials.” |
SCIENTIFIC HIGHLIGHTS
ALL CANCERS
Diazinon and Cancer Risks
The relation of diazinon, an organophosphate insecticide, with cancer risk was assessed in the Agricultural Health Study, a prospective cohort study of pesticide applicators in Iowa and North Carolina enrolled from 1993 to 1997. Among 4,961 male applicators who reported using diazinon, 301 incident cancer cases were diagnosed, and among 18,145 participants who reported no use, 968 cases were diagnosed. Based on lifetime exposure days, increased risks for the highest tertile of exposure and significant tests for trend for lung cancer and leukemia were observed. (Beane Freeman LE, Bonner MR, Blair A, Hoppin JA, Sandler DP, Lubin JH, Dosemeci M, Lynch CF, Knott C, Alavanja MC. Cancer incidence among male pesticide applicators in the Agricultural Health Study cohort exposed to diazinon. Am J Epidemiol 2005;162:1070–1079)
BREAST CANCER
Dietary Patterns
To prospectively examine the association between dietary patterns and postmenopausal breast cancer risk, data were analyzed from 40,559 women in the Breast Cancer Detection Demonstration Project, 1,868 of whom developed breast cancer. Three major dietary patterns emerged: vegetable-fish/poultry-fruit, beef/pork-starch, and traditional southern. After adjustment for confounders, there was no significant association between the vegetable-fish/poultry-fruit and beef/pork-starch patterns and breast cancer. The traditional southern pattern, however, was associated with a nonsignificantly reduced breast cancer risk among all cases (in situ and invasive) that was significant for invasive breast cancer (relative hazard = 0.78; CI = 0.65–0.95). This diet was also associated with a reduced risk in women without a family history of breast cancer, who were underweight or normal weight, or who had tumors positive for estrogen receptor or progesterone receptor. Foods in the traditional southern pattern associated with reduced risk included legumes, low-mayonnaise salad dressing, and cabbage. (Velie EM, Schairer C, Flood A, He JP, Khattree R, Schatzkin A. Empirically derived dietary patterns and risk of postmenopausal breast cancer in a large prospective cohort study. Am J Clin Nutr 2005;82:1308–1319)
COLORECTAL ADENOMAS
Hormone Therapy
Findings from some studies of colorectal cancer and adenoma suggest that the protective effect of post-menopausal hormone replacement therapy (HRT) may differ between categories of age and body mass index (BMI). The authors identified 1,468 women with at least one left-sided adenoma and 19,203 without adenoma or colorectal cancer from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Compared to never use of HRT, current use was associated with a decreased prevalence of left-sided adenoma (OR = 0.85; CI = 0.75–0.97), but there was no evidence of dose-response. The protection from current HRT use was stronger among women aged 65 or older (OR = 0.69; CI = 0.56–0.84), with a BMI less than 30 (OR = 0.82; CI = 0.71–0.95), and who regularly used aspirin or ibuprofen (OR = 0.77; CI = 0.65–0.91). The protective effect was short-lived following cessation of use. (Purdue MP, Mink PJ, Hartge P, Huang WY, Buys S, Hayes RB. Hormone replacement therapy, reproductive history, and colorectal adenomas: Data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (United States). Cancer Causes Control 2005;16:965–973)
Benzo(a)pyrene Intake
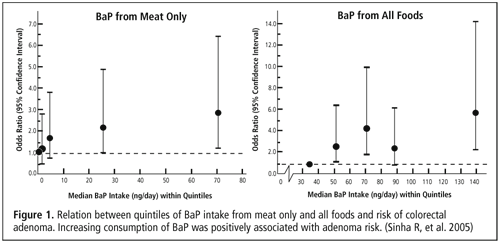
A study of 140 colorectal adenoma cases and 228 controls was conducted to address the risk associated with dietary intake of polycyclic aromatic hydrocarbons (PAH). A food-frequency questionnaire with detailed questions on meat-cooking methods and doneness levels and a benzo(a)pyrene (BaP) database (as a surrogate for total carcinogenic PAHs) were developed. The odds ratios (OR) for dietary BaP from meat with the first quintile was 1.19 (CI = 0.51–2.80) for the second quintile, 1.71 (CI = 0.76–3.83) for the third quintile, 2.16 (CI = 0.96–4.86) for the fourth quintile, and 2.82 (CI = 1.24–6.43) for the fifth quintile (p for trend = 0.01). Increased risk of colorectal adenomas was more strongly associated with BaP intake estimated from all foods (see Figure 1); the odds ratios were 2.61 (CI = 1.08–6.29) for the second quintile, 4.21 (CI = 1.79–9.91) for the third quintile, 2.45 (CI = 0.98–6.12) for the fourth quintile, and 5.60 (CI = 2.20–14.2) for the fifth quintile (p for trend = 0.002). This study provides evidence that dietary BaP plays a role in colorectal adenoma etiology. (Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev 2005;14:2030–2034)
COLORECTAL CANCER
Fiber Intake Pooled Analysis
To evaluate the controversial relationship between dietary fiber and colorectal cancer, 725,628 men and women from 13 prospective cohort studies included in the Pooling Project of Prospective Studies of Diet and Cancer were followed up for 6 to 20 years. During follow-up, 8,081 incident colorectal cancer cases were identified. For the highest vs. the lowest quintile of dietary fiber intake, an inverse association was found in the age-adjusted model (relative risk [RR] = 0.84; CI = 0.77–0.92). However, the association was attenuated and no longer statistically significant after adjusting for other risk factors (RR = 0.94; CI = 0.86–1.03). In categorical analyses, compared with dietary fiber intake of 10 to fewer than 15 g/d, the multivariate RR was 1.18 (CI = 1.05–1.31) for less than 10 g/d, and the RR was 1.00 (CI = 0.85–1.17) for 30 or more g/d. In this pooled analysis, dietary fiber intake was inversely associated with risk of colorectal cancer in age-adjusted analyses, but it was not linearly associated with risk after accounting for other dietary risk factors. (Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Hartman AM, Jacobs DR Jr, Kato I, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Dietary fiber intake and risk of colorectal cancer: A pooled analysis of prospective cohort studies. JAMA 2005;294:2849–2857)
ESOPHAGEAL CANCER
Carbonated Soft Drinks
Carbonated soft drinks (CSDs) have been associated with gastroesophageal reflux, an established risk factor for esophageal adenocarcinoma. As both CSD consumption and esophageal adenocarcinoma incidence have increased sharply in recent decades, CSD as a risk factor for esophageal and gastric cancers was examined in data from a U.S. multicenter, population-based case-control study conducted between 1993 and 1995. Contrary to the proposed hypothesis, CSD consumption three to five years before diagnosis was inversely associated with esophageal adenocarcinoma risk (highest vs. lowest quartiles, OR = 0.47; CI = 0.29–0.76; p for trend = 0.005), due primarily to intake of diet CSD. High CSD consumption did not increase risk of any esophageal or gastric cancer subtype in men or women or when analyses were restricted to nonproxy interviews. These findings indicate that CSD consumption does not likely contribute to increased incidence rates. (Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Borchardt L, Schoenberg JB, Stanford JL, West AB, Rotterdam H, Blot WJ, Fraumeni JF Jr. Carbonated soft drink consumption and risk of esophageal adenocarcinoma. J Natl Cancer Inst 2006;98:72–75)
HORMONE-RELATED CANCERS
Endometriosis and Uterine Leiomyoma
The relationships between hospital and outpatient admissions for endometriosis or leiomyomas and the development of ovarian and uterine cancers were assessed in Denmark between 1978 and 1998. In a population-based cohort study of more than 99,000 women, including 2,491 with ovarian cancers, 860 with borderline ovarian tumors, and 1,398 with uterine cancers, endometriosis seemed to predispose women to ovarian cancer, with the association restricted to endometrioid or clear cell malignancies. Five or more years after the diagnosis of endometriosis, the RRs were 2.53 (CI = 1.19–5.38) for endometrioid and 3.37 (CI = 1.24–9.14) for clear cell malignancies. Uterine leiomyomas were associated with increases in the risk of uterine malignancies, particularly sarcomas, where the RRs were 20.80 (CI = 11.32–38.22) for women with one to four years of follow-up and 5.70 (CI = 2.27–14.32) for those with more extended follow-up. (Brinton LA, Sakoda LC, Sherman ME, Frederiksen K, Kjaer SK, Graubard BI, Olsen JH, Mellemkjaer L. Relationship of benign gynecologic diseases to subsequent risk of ovarian and uterine tumors. Cancer Epidemiol Biomarkers Prev 2005;14:2929–2935)
Impact of Hysterectomy on Endometrial Cancer Rates
In the United States, endometrial carcinoma incidence rates, uncorrected for hysterectomy prevalence, are higher among white women than black women. Corrected endometrial carcinoma rates for the years from 1992 to 2000 were estimated by racial/ethnic group and age using data from the SEER program and the Behavioral Risk Factor Surveillance Survey. Hysterectomies were more prevalent among black women than among Hispanic and white, non-Hispanic women. Correcting for hysterectomy prevalence increased age-adjusted endometrial carcinoma rates per 100,000 woman-years from 29.2 to 48.7 (66.8% increase) overall, from 14.6 to 28.5 (a 95.3% increase) among blacks, from 18.8 to 29.6 (57.6% increase) among Hispanics, and from 33.2 to 54.9 (a 65.1% increase) among white non-Hispanics. This correction reduced the rate ratio for white non-Hispanics compared with blacks from 2.3 to 1.9 (see Figure 2). Among blacks but not among Hispanics or white non-Hispanics, the endometrial carcinoma risk factors of obesity and diabetes were more prevalent among hysterectomized women than among women with uteri. Failure to correct for hysterectomy prevalence may lead to underestimation of endometrial carcinoma risk, especially among blacks. The high prevalence of hysterectomy among blacks with strong endometrial cancer risk factors may partly account for lower cancer rates in this group. (Sherman ME, Carreon JD, Lacey JV Jr, Devesa SS. Impact of hysterectomy on endometrial carcinoma rates in the United States. J Natl Cancer Inst 2005;97:1700–1702)
LYMPHOMA
Anthropometry, Exercise, and Medical Conditions
In a population-based study of 1,321 HIV-negative non-Hodgkin lymphoma (NHL) cases and 1,057 controls from four Surveillance, Epidemiology, and End Results (SEER) areas, high body mass index (BMI > 35; OR = 1.73; CI = 1.15–2.59) and history of gallstones (OR = 1.95; CI = 1.11–3.40) were associated with diffuse NHL but not with follicular or all NHL combined. Height was associated with risk of all NHL combined (OR = 1.38 for > 70 vs. < 65 inches; CI = 0.98–1.94) and of both diffuse and follicular NHL. Non-occupational physical activity was inversely associated with risk of all NHL combined (ORs with increasing level: 1, 0.75, 0.71, 0.55, 0.68; p for trend = 0.04) and with risk of diffuse and follicular NHL. No associations of total energy intake, type 2 diabetes, or hypertension with NHL risk were found. Associations of BMI and history of gallstones with risk of diffuse NHL support a role for obesity. The height association results suggest a role for early life nutrition in NHL risk. (Cerhan JR, Bernstein L, Severson RK, Davis S, Colt JS, Blair A, Hartge P. Anthropometrics, physical activity, related medical conditions, and the risk of non-Hodgkin lymphoma. Cancer Causes Control 2005;16:1203–1214)
Plasma Organochlorines
Polychlorinated biphenyls (PCB) and other organochlorines and risk of NHL were the subject of this population-based U.S. case-control study. Congeners of PCBs (including coplanar congeners), dioxins, furans, and pesticides or pesticide metabolites were measured in plasma of 100 untreated cases and 100 control subjects. Certain PCB congeners were associated with increased risk of NHL, including coplanar PCBs 156, 180, and 194, with ORs for the highest vs. the lowest quartile ranging from 2.7 to 3.5 and significant trends. Each of the furan congeners was associated with risk of NHL, as were total furans, with a 3.5-fold increased risk for the highest vs. the lowest quartile and a significant trend across quartiles (p = 0.006). The toxic equivalency quotient (TEQ), a summed metric that weights congeners by their dioxin-like potency, was associated with NHL, with a 35% increased risk per 10 TEQ pg/g lipid (CI = 1.02–1.79). (De Roos AJ, Hartge P, Lubin JH, Colt JS, Davis S, Cerhan JR, Severson RK, Cozen W, Patterson DG Jr, Needham LL, Rothman N. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin’s lymphoma. Cancer Res 2005;65:11214–11226)
Immune-related Conditions and Medications
In a population-based study of 1,321 NHL cases and 1,057 controls, NHL was associated with Sjögren’s syndrome (OR = 13; CI = 1.7–100) and lupus (OR = 4.2; CI = 1.2, 15). Two NHL subtypes were strongly associated with Sjögren’s syndrome: salivary gland (OR = 290; CI = 33–2,600) and marginal zone (OR = 75; CI = 9.1–610). NHL was less strongly associated with receipt of an organ transplant (OR = 2.0; CI = 0.4–11). Corticosteroid use was unrelated to NHL, but methotrexate use was marginally associated (OR = 2.3; CI= 0.7–7.5). Family history of dermatomyositis was associated with NHL (7 cases vs. 0 controls, OR = infinite; two-sided p = 0.02), but dermatomyositis was absent in cases themselves. Results suggest that disordered immunity in some immune-related conditions can lead to NHL. (Engels EA, Cerhan JR, Linet MS, Cozen W, Colt JS, Davis S, Gridley G, Severson RK, Hartge P. Immune-related conditions and immune-modulating medications as risk factors for non-Hodgkin lymphoma: A case-control study. Am J Epidemiol 2005;162:1153–1161)
Dietary One-carbon Nutrients
The associations between NHL and folate; vitamin B2, B6, and B12; methionine; and a one-carbon antagonist, alcohol, were examined in a population-based study of 425 NHL cases and 359 controls from four SEER areas. Higher intake of one-carbon determinants from food was associated with a lower risk of NHL, but only the effects associated with vitamin B6 (highest vs. lowest quartile: OR = 0.57; CI = 0.34–0.95; p for trend = 0.01) and methionine (OR = 0.49; CI = 0.31–0.76; p for trend = 0.002) reached statistical significance. Folate from food was inversely associated with diffuse NHL (OR = 0.47; CI = 0.23–0.94; p for trend = 0.03). There was no association between total vitamins (food plus supplements) and NHL. Nonusers of alcohol had a higher NHL risk than users, and alcohol use did not modify other nutrient-NHL associations. Findings suggest that one-carbon nutrients, particularly vitamin B6 and methionine, may be protective against NHL. (Lim U, Schenk M, Kelemen LE, Davis S, Cozen W, Hartge P, Ward MH, Stolzenberg-Solomon R. Dietary determinants of one-carbon metabolism and the risk of non-Hodgkin lymphoma: NCI-SEER case-control study, 1998–2000. Am J Epidemiol 2005;162:953–964)
Incidence by WHO Subtype
A comprehensive assessment of 114,548 lymphoid neoplasms diagnosed between 1992 and 2001 in 12 SEER registries was conducted according to the WHO lymphoma classification system. Diverse incidence patterns and trends by lymphoid neoplasm subtype and population were observed. Among the elderly (aged 75 years and older), rates of diffuse large B-cell lymphoma and follicular lymphoma increased 1.4% and 1.8% per year, respectively, whereas rates of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) declined 2.1% per year. Although whites have the highest incidence of most lymphoid neoplasm subtypes, most notably for hairy cell leukemia and follicular lymphoma, blacks have a higher incidence of plasma cell and T-cell neoplasms. Asians have considerably lower rates than whites and blacks for CLL/SLL and Hodgkin lymphoma (HL). Striking differences in incidence patterns by histologic subtype strongly suggest that there is etiologic heterogeneity among lymphoid neoplasms and support the pursuit of epidemiologic analysis by subtype. (Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006;107:265–276)
TNF and IL10 Variation
The effect of common genetic variants in immune and inflammatory response genes on the risk of NHL was tested using data on 3,586 cases and 4,018 controls from eight European, Canadian, and U.S. case-control studies in the International Lymphoma Epidemiology Consortium (InterLymph). Twelve single nucleotide polymorphisms (SNPs) in nine genes that have important roles in lymphoid development, Th1/Th2 balance, and proinflammatory or anti-inflammatory pathways (IL1A, IL1RN, IL1B, IL2, IL6, IL10, TNF, LTA, and CARD15), were selected for analysis on the basis of previous functional or association data. The tumor necrosis factor (TNF)-308G-A polymorphism was associated with increased risk of NHL, particularly diffuse large B-cell lymphoma, the main histological subtype (OR = 1.29 and CI = 1.10–1.51 for GA; OR = 1.65 and CI = 1.16–2.34 for AA), but not for follicular lymphoma. The interleukin10 (IL10)-3575T-A polymorphism was also associated with increased risk of NHL, again, particularly for diffuse large B-cell lymphoma. For individuals homozygous for the TNF-308A allele and carrying at least one IL10-3575-A allele, risk of diffuse large B-cell lymphoma doubled (OR = 2.13; CI = 1.37–3.32). Common polymorphisms in TNF and IL10, key cytokines for the inflammatory response, and Th1/Th2 balance, could be susceptibility loci for NHL. (Rothman N, Skibola CF, Wang SS, Morgan G, Lan Q, Smith MT, Spinelli JJ, Willett E, De Sanjose S, Cocco P, Berndt SI, Brennan P, Brooks-Wilson A, Wacholder S, Becker N, Hartge P, Zheng T, Roman E, Holly EA, Boffetta P, Armstrong B, Cozen W, Linet M, Bosch FX, Ennas MG, Holford TR, Gallagher RP, Rollinson S, Bracci PM, Cerhan JR, Whitby D, Moore PS, Leaderer B, Lai A, Spink C, Davis S, Bosch R, Scarpa A, Zhang Y, Severson RK, Yeager M, Chanock S, Nieters A. Genetic variation in TNF and IL10 and risk of non-Hodgkin lymphoma: A report from the InterLymph Consortium. Lancet Oncol 2006;7:27–38)
BCL6 Gene Polymorphism
Exon 6 of the B-cell lymphoma 6 gene (BCL6), an oncogene that contributes to lymphomagenesis, contains a common SNP (–195 C > T; dbSNP ID: rs1056932) that alters a potential binding site for an exonic splicing enhancer. Unconditional logistic regression models were used to examine the association between this SNP and the risk of NHL in a population-based case-control study of women residing in Connecticut (including 461 case patients and 535 control subjects). The risk of NHL among women with the CC genotype was more than double that among women with the TT genotype (OR = 2.2; CI = 1.5–3.3). Higher risks were observed for B-cell chronic lymphatic leukemia/prolymphocytic leukemia/small lymphocytic lymphoma (OR = 3.5; CI = 1.6–7.8) and T-cell lymphoma (OR = 5.2; CI = 2.0–13.3). Results support the hypothesis that a genetic variant that could alter mRNA transcripts of BCL6 may contribute to the etiology of NHL. (Zhang Y, Lan Q, Rothman N, Zhu Y, Zahm SH, Wang SS, Holford TR, Leaderer B, Boyle P, Zhang B, Zou K, Chanock S, Zheng T. A putative exonic splicing polymorphism in the BCL6 gene and the risk of non-Hodgkin lymphoma. J Natl Cancer Inst 2005;97:1616–1618)
MULTIPLE MYELOMA
Monoclonal Gammopathy
The age-adjusted incidence of multiple myeloma (MM) is twice as high in African Americans as in white Americans. A few small studies have reported a higher prevalence of monoclonal gammopathy of undetermined significance (MGUS) in African Americans than in whites. The prevalence of MGUS and subsequent risk of MM were quantified among four million African American and white male veterans admitted to VA hospitals. The age-adjusted prevalence ratio of MGUS in African Americans compared to whites was 3.0 (CI = 2.7–3.3). Among 2,046 MGUS cases, the estimated cumulative risk of MM during the first 10 years of follow-up was similar for African Americans (17%) and whites (15%). In the largest study to date, results suggest that the excess risk of MM in African Americans results from an increase in risk of MGUS rather than an increased risk of progression from MGUS to MM. (Landgren O, Gridley G, Turesson I, Caporaso NE, Goldin LR, Baris D, Fears TR, Hoover RN, Linet MS. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood 2006;107(3):904–906)
NASOPHARYNGEAL CANCER
Killer Cell Immunoglobulin-like Receptors and HLA-C Genes
Nasopharyngeal carcinoma (NPC), an Epstein-Barr virus (EBV)-associated malignancy, is associated with specific human leukocyte antigen alleles that present viral and other antigens to the immune system. To determine whether innate immunity is associated with NPC, a case-control study was conducted among 295 Taiwanese NPC cases (99% EBV seropositive) and 252 community controls (29% EBV seropositive). Results suggested that an increasing number of activating killer cell immunoglobulin-like receptor (KIR) alleles was associated with increasing NPC risk, particularly among individuals seropositive for anti-EBV antibodies known to be linked to NPC susceptibility (p for trend = 0.07). Among EBV-seropositive individuals, carriers of five or more activating KIRs had a 3.4-fold increased risk of disease (CI = 0.74–15.7) compared with those having no functional activating KIRs. There was no clear evidence of risk associated with increasing numbers of inhibitory KIRs. (Butsch Kovacic M, Martin M, Gao X, Fuksenko T, Chen CJ, Cheng YJ, Chen JY, Apple R, Hildesheim A, Carrington M. Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2005;14:2673–2677)
PANCREATIC CANCER
Insulin Resistance and Serum Insulin and Glucose
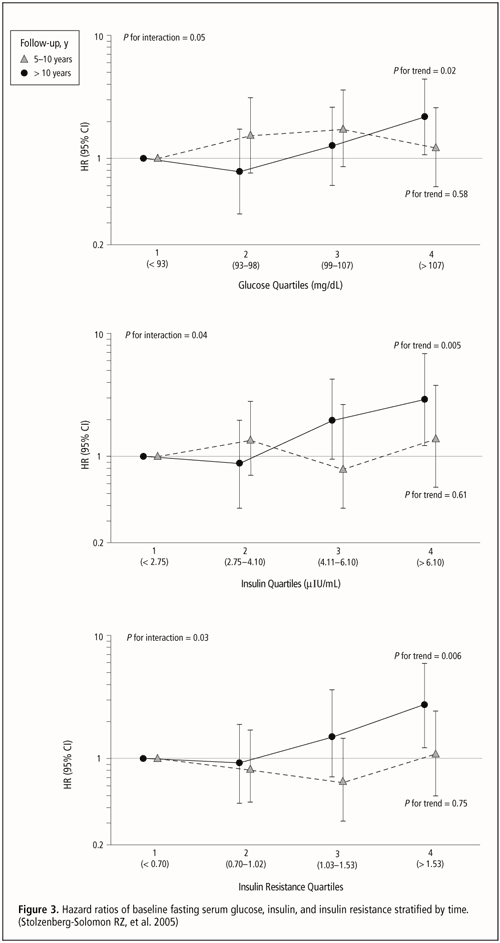
Obesity, diabetes mellitus, and glucose intolerance have been associated with increased pancreatic cancer risk, but prediagnostic serum insulin concentration has not been evaluated as a predictor of this malignancy. The relationships of prediagnostic fasting glucose and insulin concentrations and insulin resistance to incidence of exocrine pancreatic cancer were examined in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study of 29,133 male Finnish smokers, including 169 incident pancreatic cancer cases and 400 controls. After adjusting for age, smoking, and body mass index, insulin resistance and higher baseline fasting serum concentrations of glucose and insulin were positively associated with pancreatic cancer. The presence of biochemically defined diabetes mellitus (glucose > or = 126 mg/dL) and insulin concentration in the highest vs. the lowest quartile both showed a significant two-fold increased risk (hazard ratio [HR] = 2.13; CI = 1.04–4.35 and HR = 2.01; CI = 1.03–3.93, respectively). The positive associations were stronger among the cases that occurred more than 10 years after baseline (highest vs. lowest quartile: glucose: HR = 2.16; CI = 1.05–4.42, insulin: HR = 2.90; CI = 1.22–6.92, and insulin resistance: HR = 2.71; CI = 1.19–6.18) (see Figure 3). Results support the hypothesis that higher insulin concentrations and insulin resistance predict the risk of exocrine pancreatic cancer. (Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872–2878)
PROSTATE CANCER
Meat and Meat Mutagens
The association between meat and meat mutagens—specifically, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)—and prostate cancer risk was investigated in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. During follow-up, a total of 1,338 prostate cancer cases were diagnosed among 29,361 men; this included 868 incident diagnoses and 520 advanced cases. Neither intake of red meat or white meat, or all meat combined, was associated with prostate cancer risk. Individuals who consumed more than 10 g/d of very well-done meat, compared to those who did not consume very well-done meat, were associated with a 1.4-fold increased risk of prostate cancer (CI = 1.05–1.92) and a 1.7-fold increased risk (CI = 1.19–2.40) of incident disease. The highest quintile of PhIP was associated with a 1.2-fold increased risk of prostate cancer (CI = 1.01–1.48) and a 1.3-fold increased risk of incident disease (CI = 1.01–1.61). This study lends epidemiologic support to animal studies that have implicated PhIP as a prostate carcinogen. (Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, Sinha R. A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res 2005;65:11779–11784)
HSD17B1 Gene
The association between the steroid hormone gene HSD17B1 and prostate cancer was investigated by the Breast and Prostate Cancer Cohort Consortium. Researchers systematically characterized variation in HSD17B1 by targeted resequencing and dense genotyping, selected haplotype-tagging single nucleotide polymorphisms (htSNPs) that efficiently predict common variants, and genotyped these htSNPs in 8,290 prostate cancer cases and 9,367 controls. HSD17B1 htSNPs or htSNP haplotypes were not associated with risk of prostate cancer or tumor stage. One haplotype was inversely associated with risk of prostate cancer among Latinos and Japanese Americans but not among African Americans, Native Hawaiians, or whites. Results suggest that germline variants in HSD17B1 characterized by these htSNPs do not substantially influence the risk of prostate cancer in whites. (Kraft P, Pharoah P, Chanock SJ, Albanes D, Kolonel LN, Hayes RB, Altshuler D, Andriole G, Berg C, Boeing H, Burtt NP, Bueno-de-Mesquita B, Calle EE, Cann H, Canzian F, Chen YC, Crawford DE, Dunning AM, Feigelson HS, Freedman ML, Gaziano JM, Giovannucci E, Gonzalez CA, Haiman CA, Hallmans G, Henderson BE, Hirschhorn JN, Hunter DJ, Kaaks R, Key T, Marchand LL, Ma J, Overvad K, Palli D, Pike MC, Riboli E, Rodriguez C, Setiawan WV, Stampfer MJ, Stram DO, Thomas G, Thun MJ, Travis R, Trichopoulou A, Virtamo J, Wacholder S. Genetic variation in the HSD17B1 gene and risk of prostate cancer. PLoS Genet 2005;1:e68)
SECOND CANCERS
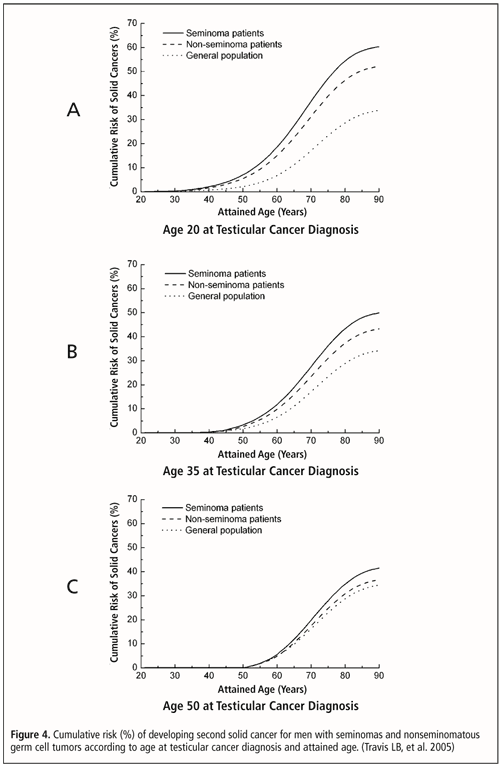
Testicular Cancer Survivors
Although second primary cancers are a leading cause of death among men with testicular cancer, few studies have quantified risks among long-term survivors. Within 14 population-based tumor registries in Europe and North America (1943–2001), 40,576 one-year survivors of testicular cancer were identified and data ascertained on any new incident solid tumors among these patients. A total of 2,285 second solid cancers were reported in the cohort. The relative risk and excess absolute risk decreased with increasing age at testicular cancer diagnosis (see Figure 4). Among 10-year survivors diagnosed with testicular cancer at age 35, the risk of developing a second solid tumor was increased (RR = 1.9; CI = 1.8–2.1). Risk remained elevated for 35 years (RR = 1.7; CI = 1.5–2.0; p < .001). Statistically significantly elevated risks were observed, for the first time, for cancers of the pleura (RR = 3.4; CI = 1.7–5.9) and esophagus (RR = 1.7; CI = 1.0–2.6). Cancers of the lung (RR = 1.5; CI = 1.2–1.7), colon (RR = 2.0; CI = 1.7–2.5), bladder (RR = 2.7; CI = 2.2–3.1), pancreas (RR = 3.6; CI = 2.8–4.6), and stomach (RR = 4.0; CI = 3.2–4.8) accounted for almost 60% of the total excess risk. Overall patterns were similar for seminoma and nonseminoma patients, with lower risks observed for nonseminoma patients treated after 1975. For patients diagnosed with seminomas or nonseminomatous tumors at age 35, cumulative risks of solid cancer by age 75 were 36% and 31%, respectively, compared with 23% for the general population. Testicular cancer survivors are at increased risk of solid tumors for at least 35 years after treatment. (Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst 2005;97:1354–1365)
Breast Cancer after Hodgkin Lymphoma
The risk of breast cancer after treatment for HL at a young age was estimated among 3,817 female one-year survivors of HL diagnosed at age 30 or younger between 1965 and 1994. Cumulative absolute risks of breast cancer increased with age at the end of the follow-up, time since HL diagnosis, and radiation dose. For an HL survivor who was treated at age 25 with a chest radiation dose of at least 40 Gy without alkylating agents, estimated cumulative absolute risks of breast cancer by ages 35, 45, and 55 years were 1.4% (CI = 0.9%–2.1%), 11.1% (CI = 7.4%–16.3%), and 29.0% (CI = 20.2%–40.1%), respectively. Cumulative absolute risks were lower among women treated with alkylating agents. These estimates are applicable to HL survivors treated with regimens of the past and can be used to counsel such patients and plan management and preventive strategies. (Travis LB, Hill D, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Pukkala E, Lynch CF, Pee D, Smith SA, Van’t Veer MB, Joensuu T, Storm H, Stovall M, Boice JD Jr, Gilbert E, Gail MH. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst 2005;97:1428–1437)
TESTICULAR CANCER
Incidence among Black Men
Since at least the beginning of the SEER program in 1973, the incidence rates for testicular germ cell tumors (TGCT) have been five times higher among white men than among black men. In addition, rates among white men have been increasing, whereas rates among black men were stable through 1988. However, a recent examination of ethnic-specific rates suggested that the incidence among black men may have begun to change in the 1990’s. Between the periods 1988 to 1992 and 1998 to 2001, the incidence of TGCT among black men increased by 100%, with the incidence of seminoma increasing twice as much (124.4%) as the incidence of nonseminoma (64.3%). Over the period from 1973 to 2001, there was no evidence of a change in the proportion of tumors diagnosed at earlier stages among black men. In contrast, the proportion of tumors diagnosed at localized stages significantly increased among white men. Although the reasons for the increase in TGCT among blacks are unclear, screening and earlier diagnosis of TGCT do not seem to be factors. (McGlynn KA, Devesa SS, Graubard BI, Castle PE. Increasing incidence of testicular germ cell tumors among black men in the United States. J Clin Oncol 2005:23:5757–5761)
DCEG PEOPLE IN THE NEWS
 |
Blanche Alter, M.D., M.P.H., Clinical Genetics Branch (CGB), received a Founder Award from the Fanconi Anemia Research Fund Scientific Symposium. Between September and December, Dr. Alter spoke about her research on inherited bone marrow failure syndromes at meetings in Geneva, Toronto, Prague, Israel, Baltimore, and Washington, DC. William Anderson, M.D., M.P.H., Biostatistics Branch (BB), gave an invited talk titled “Hierarchical (stem cell) versus stochastic breast cancer models” at the George Washington University Medical Center in Washington, DC, in September. He later delivered a talk on “Cracks in the breast cancer paradigm” at the Uniformed Services University of the Health Sciences in Bethesda in October. |
 |
Aaron Blair, Ph.D., Occupational and Environmental Epidemiology Branch (OEEB), gave an invited presentation on “Occupational exposures and cancer” at the Penn State Cancer Institute in Hershey in October. In October, Louise Brinton, Ph.D., Chief of the Hormonal and Reproductive Epidemiology Branch (HREB), gave talks on cancer and endometriosis for medical and lay audiences at the 25th Anniversary Conference of the Endometriosis Association in Milwaukee. She also spoke to the New York Obstetrical Society in New York City in December on “Controversies regarding iatrogenic causes of female cancers.” |
 |
Philip Castle, Ph.D., M.P.H. (HREB), spoke about “HPV testing to reduce cervical cancer burden” at the Infectious Disease Conference held in Charleston, West Virginia in October. He also gave a talk on “Cervical cancer: Epidemiology and prevention” for the NIH Academy in Bethesda in November. Wong-Ho Chow, Ph.D. (OEEB), gave a presentation on “Epidemiologic studies of esophageal adenocarcinoma: New findings and future plans” at the Second Esophageal Cancer and Barrett’s Metaplasia Research Summit in Las Vegas in November. |
 |
Mitchell Gail, M.D., Ph.D. (Chief of BB), delivered a presentation titled “Using case-control and case-only probands with family history to estimate genotype-specific cumulative risk” at the Joint Statistical Meetings in Minneapolis in August. He also gave a talk on “Assessing absolute individualized breast cancer risk” at Fox Chase Cancer Center in Philadelphia in October. Mark Greene, M.D. (Chief of CGB), presented a talk on “Genetics of ovarian cancer” at the Symposium on Breast and Gynecological Malignancies at the King Hussein Cancer Center in Amman, Jordan in September. At the Ovarian Cancer Symposium at the University of Pittsburgh Cancer Institute in October, Dr. Greene presented “Prevention and early detection of ovarian cancer among women at increased genetic risk,” and Patricia Hartge, Sc.D., Deputy Director of the Epidemiology and Biostatistics Program (EBP), presented “Screening for ovarian cancer in high risk women.” Michael Hauptmann, Ph.D. (BB), taught a two-day course in genetic epidemiology for students in the public health program at the University of Munich in November. He also gave an invited presentation on “The NCI cohort of workers in formaldehyde industries” at the Annual European Meeting of the Toxicology Forum in Brussels in November. Richard Hayes, D.D.S., Ph.D. (OEEB), was an invited lecturer at the Collegium Ramazzini conference, Framing the Future in Light of the Past: Living in a Chemical World, in September in Bologna, Italy. Dr. Hayes informed attendees about “Benzene and cancer risk in China.” |
 |
Robert Hoover, M.D., Sc.D., Director of EBP, was invited by the Princess Takamatsu Cancer Research Fund to make a presentation on “Occupational and environmental contaminants and cancer” at its 36th International Symposium, Developments in Cancer Epidemiology: Prospects for Cancer Control in the Asian Pacific, held in Tokyo in November. In November, Ann Hsing, Ph.D. (HREB), gave an invited talk on “Prostate cancer screening in Ghana” at the International Conference on Cancer in Africa in Dakar, Senegal and chaired a session on “Cancer research and training in Africa.” |
 |
Peter Inskip, Sc.D., Radiation Epidemiology Branch (REB), spoke on the etiology of brain tumors at the Second Neuro-Oncology Update: State of the Art 2005 at Winship Cancer Institute, Emory University School of Medicine in Atlanta in September; the Hematology-Oncology Grand Rounds at Josephine Ford Cancer Center in Detroit in October; and at the Tumors of the Central Nervous System Course, Brigham and Women’s Hospital in Boston in November. Daehee Kang, M.D., Ph.D. (OEEB), organized the Asian Cohort Consortium meeting held in Seoul, South Korea in September. Ola Landgren, M.D., Ph.D., Genetic Epidemiology Branch (GEB), spoke on “Risk of MGUS and subsequent multiple myeloma among African American and White veterans in the United States” and “Familial risk of autoimmune and hematopoietic diseases in families of patients with multiple myeloma: Population-based case-control studies in Scandinavia” at the Nordic Myeloma Study Group in Copenhagen in November. |
 |
Martha Linet, M.D., M.P.H. (Chief of REB), was an organizer of the Workshop to Establish an International Childhood Cancer Cohort Consortium held in September in Rockville, Maryland. The workshop was supported by the NIH Office of Rare Diseases, the National Children’s Study, and the U.S. Environmental Protection Agency. Principal investigators of cohort studies of children in Denmark, Norway, China, the United Kingdom, France, Spain, Australia, and Israel met to establish an international consortium to address specific promising hypotheses that cannot be easily studied in case-control studies. |
 |
Sam Mbulaiteye, M.D., Viral Epidemiology Branch (VEB), recently gave two invited talks: “Epidemiology of AIDS-related malignancies” at the 27th Annual Conference of the International Association of Cancer Registries in Entebbe, Uganda in September and “Record-linkage as a tool to study cancer incidence in people with AIDS in Africa: The Uganda AIDS cancer match registry study” at Case Western Reserve University in November. Roxana Moslehi, Ph.D., M.S. (BB), gave an invited two-part presentation at George Washington University in September. The presentations were titled “Genetic epidemiology: Definition and strategies” and “Genetic epidemiologic studies of breast and ovarian cancers: Controversies and challenges.” In addition, Dr. Moslehi and Mark Purdue, Ph.D. (OEEB), have been appointed to represent the fellows on the DCEG Office of Education Advisory Group Committee. In December, Thomas O’Brien, M.D., M.P.H. (HREB), gave a presentation entitled “Epidemiology of human liver cancer” as part of the symposium, Inflammation and Liver Cancer: The Role of the Microenvironment in Tumor Progression, held at the NIH. June Peters, M.S., C.G.C. (CGB), chaired a session of presentations on “Genetic counseling, testing, and ethics” at the American Society of Human Genetics meeting in Salt Lake City, in October, and she convened and presented on a panel on family communications at the National Society of Genetic Counselors Short Course on Risk Perception and Communication in Los Angeles in November. |
 |
Ruth Pfeiffer, Ph.D. (BB), delivered a biostatistics seminar titled “Inference for covariates from combined family and case-control data” at Memorial Sloan-Kettering Cancer Center in New York City in October. In addition, Dr. Pfeiffer was an invited speaker at the 23rd Seminar of the Austrian-Swiss Region of the International Biometric Society in Graz, Austria in September. Panagiota Mitrou, Ph.D., Nutritional Epidemiology Branch (NEB), gave an invited talk on “Intakes of calcium, dairy products, and prostate cancer risk in the ATBC study” at the AACR Frontiers in Cancer Prevention Research conference in Baltimore in November. |
 |
Cécile Ronckers, Ph.D. (REB), gave a talk entitled “Dose response analysis in radiation epidemiology: Breast and thyroid cancer” at the Julius Center for Health Sciences and Primary Care Seminar Series at the Utrecht University Medical Center in the Netherlands in September. |
 |
Philip Rosenberg, Ph.D. (BB), gave a presentation titled “The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy” to the 47th Annual Meeting of the American Society of Hematology in Atlanta in December. |
 |
Lois Travis, M.D., Sc.D. (REB), spoke on cancer survivorship at the 55th Annual Meeting of the American Society of Human Genetics in Salt Lake City in October and the ASCO-Institute of Medicine-National Coalition of Cancer Survivorship Symposium in Washington, DC, in November. She also spoke on “The epidemiology of second primary cancers” at the AACR Frontiers in Cancer Prevention Research conference in Baltimore in November. At a Keystone Symposium, Advances in the Understanding and Treatment of Melanoma, held in Santa Fe in January, Margaret Tucker, M.D. (Chief of GEB and Director of the Human Genetics Program), gave a talk entitled “Who is at risk of melanoma?” Jim Vaught, Ph.D., Office of the Director, spoke at the International Environmental Specimen Banking Symposium in November in Charleston, South Carolina on “The International Society for Biological and Environmental Repositories.” He also presented on “Biospecimen collection, processing, storage, and dissemination” at the NCI Symposium on International Harmonization of Biorepository Practices in November in Washington, DC. In September, Sophia Wang, Ph.D. (HREB), gave talks on “Genetic susceptibility to smoking and obesity” at the University of Minnesota, Twin Cities, and on “Family history of hematopoietic malignancies and risk for non-Hodgkin lymphoma” at the International Familial Chronic Lymphocytic Leukemia Consortium in New York. In October, Dr. Wang spoke on “Susceptibility genes in etiologic extremes: Cervical cancer and non- Hodgkin lymphoma” at the Mayo Clinic in Rochester, Minnesota. Margaret Wright, Ph.D. (NEB), gave a talk on “Risk factors for prostate and bladder cancers: Emerging insights” at the University of Texas Health Science Center at San Antonio in October. |
THIRD MEETING OF THE INTERNATIONAL FCLL CONSORTIUMThe third meeting of the International Familial Chronic Lymphocytic Leukemia (FCLL) consortium was held in Brooklyn in September. Attendees from DCEG included Neil Caporaso, M.D. (GEB), David Ng, M.D. (GEB), Sophia Wang, Ph.D. (HREB), Lynn Goldin, Ph.D. (GEB), and Ola Landgren, M.D., Ph.D. (GEB). Other luminaries attending the meeting included Carlo Croce, M.D. (Ohio State University), Andy Rawstron, Ph.D. (University of Leeds), Paolo Ghia, M.D., Ph.D (University of Turin), Nick Chiorazzi, M.D. (North Shore Long Island Jewish Research Institute), Bob Vogt, Ph.D. (CDC), Professor Richard Houlston (Institute of Cancer Research, Sutton, Surrey), Susan Slager, Ph.D. (Mayo Clinic), and Gerald Marti, M.D., Ph.D (FDA). The meeting was organized by Drs. Caporaso and Goldin, along with Drs. Marti and Slager. The FCLL consortium is dedicated to coordinating international efforts to investigate this disease and related conditions, including monoclonal B-cell lymphocytosis, a precursor state that has been identified in apparently unaffected members of high risk kindreds. |
|
COMINGS . . . GOINGS
 |
Jiyoung Ahn, Ph.D., has joined the Nutritional Epidemiology Branch (NEB) as a visiting fellow. Dr. Ahn earned a Ph.D. in nutritional sciences from Cornell University, with minors in epidemiology and toxicology. For her dissertation, she investigated gene-diet interactions in relation to breast cancer risk and prognosis. Dr. Ahn plans to work with Demetrius Albanes, M.D., and other investigators to study genetic susceptibility to prostate cancer. Jinbo Chen, Ph.D., M.S., a research fellow in the Biostatistics Branch (BB), left DCEG at the end of December to take a position as Assistant Professor at the University of Pennsylvania in the Biostatistics Department of the School of Medicine. |
 |
Jonine Figueroa, Ph.D., M.P.H., has joined the Hormonal and Reproductive Epidemiology Branch (HREB) as a Cancer Prevention Fellow of the Division of Cancer Prevention (DCP). She recently completed her M.P.H. at the Columbia University Mailman School of Public Health. Prior to entering the DCP Fellowship Program, she completed a Ph.D. in molecular genetics and microbiology at Stony Brook University in New York. Dr. Figueroa is working with Montserrat Garcia-Closas, M.D., Ph.D., on genetic susceptibility to bladder cancer. Marc Gunter, Ph.D. (NEB), has joined the faculty of the Albert Einstein College of Medicine in New York City in the Department of Epidemiology and Population Health. Michael Hauptmann, Ph.D., an investigator in BB, has taken a position as a senior statistician at the Netherlands Cancer Institute in Amsterdam. |
 |
Nicole Keesecker, M.A., has joined DCEG as a Health Communication Intern. Ms. Keesecker is a student at Michigan State University, where she expects to complete an M.A. in Health Communication this spring. Her undergraduate degree in biology is from the University of Illinois College of Pharmacy. She has worked in the Michigan Office of the Surgeon General and at Michigan State University. |
 |
Larissa Korde, M.D., M.P.H., has joined the Clinical Genetics Branch (CGB) as a Staff Clinician. Dr. Korde is board-certified in internal medicine and medical oncology and has just completed a DCP Cancer Prevention Fellowship, which included an M.P.H. in biostatistics and epidemiology from George Washington University. |
 |
Jill Koshiol, Ph.D., has joined the Genetic Epidemiology Branch (GEB) as a postdoctoral fellow. Dr. Koshiol received her Ph.D. in epidemiology from the University of North Carolina at Chapel Hill in 2005 and joined the NCI as a DCP Cancer Prevention Fellow last July. She will be working with Philip Taylor, M.D., Sc.D. |
 |
Deukwoo Kwon, Ph.D., joined the Radiation Epidemiology Branch (REB) in October as a postdoctoral fellow. Dr. Kwon holds a Ph.D. in statistics from Texas A&M University. While at REB, Dr. Kwon will work on uncertainty analysis and measurement error in modeling the radiation dose-response relation for the U.S. Radiologic Technologists Study and for the genetics component of that study. |
 |
Kyoung-Mu Lee, Ph.D., has joined the Occupational and Environmental Epidemiology Branch (OEEB) as a visiting fellow. He received an M.S. in environmental health sciences in 2000 and a Ph.D. in epidemiology from the Seoul National University in South Korea in 2005. His research has focused on studies of gene-environment interactions for breast and lung cancer and the etiology of childhood cancers. |
 |
Ursula Leitzmann, M.S., M.A., formerly Program Manager for REB, has left NIH to pursue a career in intercultural training at IOR Global Services. While working at NCI, Ms. Leitzmann completed a master’s degree in intercultural relations and started a consulting business focusing on training in intercultural relations. She taught classes in DCEG and across NIH entitled “Cultural diversity at NIH” and “Cultural competence in health care.” Jolanta Lissowska, Ph.D., has returned to the Cancer Center and Institute of Oncology in Warsaw, Poland after completing a year with HREB as a guest scientist. During her year with HREB, she collaborated with DCEG investigators on a multidisciplinary study of breast, ovarian, and endometrial cancers in Poland. |
 |
Sue Kyung Park, M.D., Ph.D., an HREB postdoctoral fellow since 2004, has accepted an associate professorship with the Department of Preventive Medicine, Seoul National University College of Medicine in South Korea. Cécile Ronckers, Ph.D., a research fellow in REB, has accepted a position as senior epidemiologist and coordinator of a nationwide follow-up study of childhood cancer survivors at the Department of Pediatric Oncology of the Academic Medical Center of Amsterdam. |
 |
Gilles Thomas, Ph.D., M.D., has joined DCEG as a Senior Scientist in the Core Genotyping Facility. A distinguished scientist in the field of genetics and genomics, Dr. Thomas was previously at the Fondation Jean Dausset-CEPH and the Université Paris VI. He received a Ph.D. in biochemistry from the Université Paris VII in 1977 and an M.D. from the Université Paris VI in 1989. He is the senior investigator for the CGEMS (Cancer Genetic Markers of Susceptibility) project. Fan-Chen Tseng, Ph.D., a DCEG postdoctoral fellow since 2002, has accepted a position as a Research Associate in the Clinical Research Division at the National Research Institute of Health of Taiwan. |
 |
Kai Yu, Ph.D., has joined BB as a tenure-track investigator. Dr. Yu received his Ph.D. from the University of Pittsburgh and conducted postdoctoral research at Stanford University. He worked as a statistical geneticist at a biopharmaceutical company in Cambridge, Massachusetts before becoming a Research Assistant Professor in the Division of Biostatistics at Washington University in St. Louis. Dr. Yu’s main research interest is in statistical methods for genetic epidemiology. |
 |
Ying-Ying Yu, M.S., has joined the Viral Epidemiology Branch as a predoctoral fellow. She has an M.S. in statistics from the University of Memphis and is currently in a Ph.D. program in epidemiology at the University of Maryland School of Medicine. Fang Fang Zhang, M.D., a predoctoral fellow at OEEB, completed her fellowship at the end of August. Dr. Zhang is continuing her doctoral research at the Columbia University Mailman School of Public Health. |
WRITING WORKSHOPS PROMOTE TEAMWORKThis winter, approximately 35 DCEG staff members participated in a writing improvement program. The program, according to Sue K. Harris of Strategic Marketing and Business Communications, “clarifies and reviews writing techniques needed to produce writing that is organized, clear, succinct, positive and persuasive. At the same time it empowers writers and reviewers to successfully carry out their unique roles during the writing process.” Separate sessions were offered for reviewers (mentors) and writers (fellows and other scientists). The half-day session for reviewers focused on how to identify problem areas and give specific feedback to help writers develop their skills. The program emphasized manuscripts, research proposals, and other professional documents. Writers attended four half-day sessions that presented a systematic approach to improving writing and editing skills. Topics included assignment clarification, effective writing techniques, unity and coherence, clarity, and techniques to reduce wordiness. At the end of the program, the writers were given the opportunity to have two samples of their writing analyzed. The instructor met with participants individually to give feedback. The course instructor, Ms. Harris, presented a comprehensive program of writing improvement aimed at fostering teamwork between writers and reviewers. Shelia Zahm, Sc.D., DCEG Deputy Director, participated in the session for reviewers and found the course to be valuable. “Sue Harris provided excellent ideas on how to streamline the writing and editing process, which are especially helpful in a ‘team-science’ environment where papers have multiple authors, who may not always agree. More organizing, outlining, and discussion before writing are key,” Dr. Zahm said. |
REB EXPERTISE AIDS NATIONAL EFFORT AGAINST RADIOLOGICAL TERRORISM
The Radiation Epidemiology Branch (REB) has been tasked with expanding, enhancing, and developing new ways to contribute to the national strategic plan and research agenda for medical countermeasures against radiological and nuclear threats. As part of a joint program with the U.S. Department of Health and Human Services and the Office of Public Health Emergency Preparedness, NIH is playing a major role in evaluating the potential impacts of these threats on human health. At NIH, the program is coordinated by the National Institute of Allergy and Infectious Diseases, with help from experts in several areas of NCI.
Among the broad and complex types of potential radiological and nuclear threats are detonation of conventional explosives combined with radioactive materials (e.g., “dirty bombs”), clandestine placement of radioactive sources in public places, contamination of food and water supplies, attacks on nuclear reactors or sites where radioactive materials are produced or stored, and detonation of a nuclear explosive device.
REB investigators will be focusing their efforts in four major areas. Foremost is the Branch’s long-standing expertise in dosimetry. Because most current biodosimetry methods are expensive and time-consuming, REB proposes to develop an alternative and less expensive dose estimation method, which combines data about radiation levels in and around a source with knowledge about the location of individuals during and following an event. To provide important data on thyroid and other organ doses associated with higher fallout-related radiation levels, REB investigators will draw on the wealth of knowledge derived from studies in Chornobyl, Kazakhstan, and the Marshall Islands.
The Branch’s epidemiological studies may provide valuable information about late effects of radiation among people exposed at various ages and those tasked with cleaning up the debris from radiological terrorist events.
Other dosimetry-related REB activities will include expanded efforts to evaluate the prevalence of radiation-associated chromosomal aberrations in offspring of women exposed to high environmental levels of radionuclides. In addition, the Branch is collaborating to evaluate the reliability of electron paramagnetic resonance measurements made on tooth enamel to help refine environmental dose models.
REB members are also collaborating on the development of optically stimulated luminescence of tooth enamel in the hopes of providing a quick, easy, and inexpensive approach for assessing radiation exposure in vivo. If the required sensitivity can be reached, this new method would be invaluable in triage and dose reconstruction in large populations. Recently, REB researchers initiated biokinetic studies to more accurately estimate radiation doses to the thyroid from I-131, or radioactive iodine, in a cohort study of patients with thyrotoxicosis. A potentially important application of this work would be to assess radiation doses to the thyroid of young persons with thyroid abnormalities who are exposed to accidental or intentional release of radioiodines.
As a second major contribution, the Branch’s epidemiological studies may provide valuable information about late effects of radiation among people exposed at various ages and those tasked with cleaning up the debris from radiological terrorist events. The epidemiological studies in Chornobyl and Kazakhstan are sources of potential estimates of risk for cancer and other chronic diseases after exposure to fallout.
A third useful contribution is Branch members’ ongoing work in identifying and quantifying the sources of uncertainty in dose and risk estimation. Understanding sources of uncertainty is the first step to increasing the accuracy of risk estimation.
Finally, the fourth major emphasis of REB’s efforts will be the training of a new generation of scientists in radiation epidemiology, dosimetry, and relevant methods of statistical analysis.







 Dr.
Fraumeni has continued to express his appreciation for the contributions
that COS has made to the Division over the years. “Mary
Lou McMaster served with great distinction as Committee Chair
and provided me and the senior leadership with invaluable counsel,” Dr.
Fraumeni said. “The committee has been brilliant at identifying
problems at the earliest stage, thus making it possible to take
corrective steps that enrich the work life in the Division and
empower staff to maximize their scientific potential.”
Dr.
Fraumeni has continued to express his appreciation for the contributions
that COS has made to the Division over the years. “Mary
Lou McMaster served with great distinction as Committee Chair
and provided me and the senior leadership with invaluable counsel,” Dr.
Fraumeni said. “The committee has been brilliant at identifying
problems at the earliest stage, thus making it possible to take
corrective steps that enrich the work life in the Division and
empower staff to maximize their scientific potential.”