|
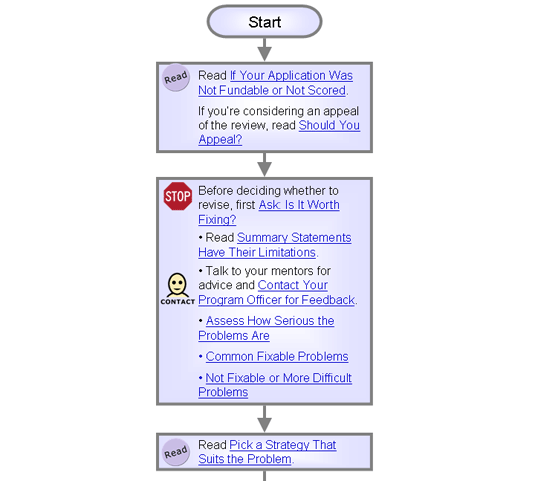 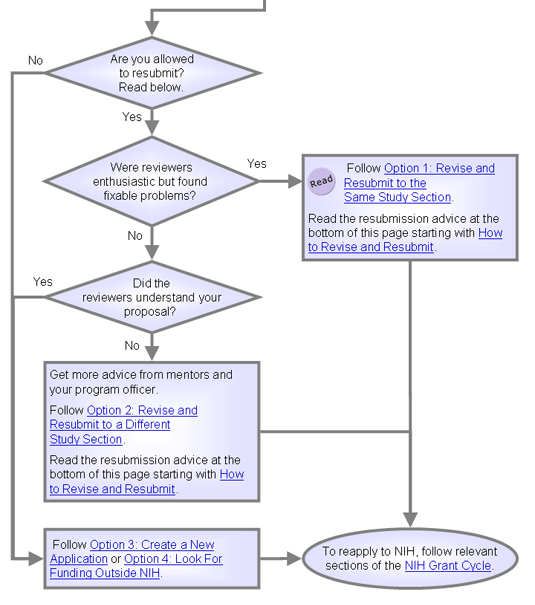
Table of Contents
Note: Because this document is almost entirely advice, we did not mark sections with the "Our advice" label used elsewhere in the tutorials.
Are You Ready for This Part?
Part 11b. Not Funded, Reapply advises you how to reapply if you didn't receive funding.
Before reading this page, be sure that you . . .
If Your Application Was Not Fundable or Not Scored
What if you submit a grant application to NIH and it does not get funded? You're in good company!
| Wait until you can address the matter calmly and objectively before deciding what to do next. |
|
Competition is Tough
Competition is tough, and it is very common not to succeed at the first attempt. More people succeed on their second try than on their first, and still more succeed on their third attempt.
Still, when you hear the news, you'll probably feel angry at being rejected, and you may feel that some of the criticism from the reviewers is off the mark. It very well may be.
First, deal with the rejection. Wait until you can address the matter calmly and objectively before deciding what to do next. Sending an angry letter to a study section or an institute would not be productive.
Next, figure out which strategy is best for you. Various approaches work best in different situations, as we describe below.
If Your Application Was Not Scored
Because unscored applications don't benefit from a full review, it's much harder to get a sense of the reviewers' appraisal of their merit. Some unscored applications may be of higher scientific merit than others that received a score.
Why? In today's budget environment, high-quality applications might be unscored because the pool contains a lot of outstanding applications, including resubmissions, which have already addressed the study section's concerns.
If your application is unscored, stay objective. Spend time figuring out what areas reviewers felt had problems, and follow our advice on revising below.
Should You Appeal?
Though you can appeal a review you feel was seriously flawed, we strongly advise against it.
Consider the following:
| We strongly advise you not to appeal. |
|
- You can appeal only for defects in the review procedure, for example, conflict of interest or bias, not differences of scientific opinion.
- Appealing wastes time.
- NIAID's Council considers all formal appeals, but members very rarely overturn review results.
- Even if you win, you will have to resubmit the application for the next review cycle.
- If you still want to appeal, contact your program officer.
- Read more in our Appeals of Scientific Review of Grant Applications SOP.
 |
Given the advice above, do you want to appeal?
|
Ask: Is It Worth Fixing?
Each unfunded application falls into one of three categories:
| If the problems are fixable, revise the application. |
|
- Has fixable problems.
- Is fatally flawed.
- Does not generate reviewer enthusiasm (dull topic).
Before you can decide what to do, you need to determine whether the application is worth fixing, its faults are fixable, and the reviewers were appropriate.
Spend some time analyzing results and gathering as much feedback as you can from the summary statement, your program officer, and senior investigators at your institution.
Try to determine whether the reviewers seemed enthusiastic about your idea. Ask brutally honest colleagues to assess the reviewers' level of interest in the summary statement. Also ask your program officer for a sense of reviewer interest.
If you decide to revise and resubmit after reading the information below, NIH allows you to resubmit your application once. You may be able to start revising even before you get your summary statement -- for more details now, read Resubmission Timing below.
Summary Statements Have Their Limitations
| Summary statements hit the highlights as far as the review progressed. |
|
After you get your summary statement, read it carefully and analytically. Show it to colleagues for their interpretation.
You can correct all the problems in the summary statement and still not get a fundable score when you reapply.
Why? A summary statement is not a complete guide to fixing your application:
- It does not detail every problem but hits the highlights as far as the review progressed.
- Once reviewers find a "fatal flaw," they may stop discussing the application because time is short.
- The flaw could be something simple to correct such as not describing how you plan to protect the safety of lab workers or animals, or it may be a fatal flaw such as an unprovable hypothesis.
- Once the reviewers stop discussing the application, their feedback ends, and you have no way of knowing what they may have found had they continued.
- When you resubmit, the next initial peer review panel could have new reviewers who see your project differently. These reviewers may identify new problems.
- If overall enthusiasm for the proposal is low, no amount of revising will help, even if you address the points in the summary statement.
- For more background now, see Know What a Summary Statement Means.
Contact Your Program Officer for Feedback
| Your program officer may be able to give you more insight into the peer review discussion if he or she attended the meeting. |
|
Contact your program
officer to see if you can
get more feedback from the review. NIAID program staff often
attend review meetings
as observers.
- If the program officer was at the meeting, he or she may be able to give you more insight into the discussion.
- Be sure to ask about the level of reviewer enthusiasm for your idea and points not addressed in the summary statement.
Also ask your program officer about your chances of funding.
- If your application received a percentile or priority score that's not fundable now, your program officer will discuss your options with you.
- Your application may be on a list for possible selective pay or R56-Bridge award (or both) funds or deferred until the end of the fiscal year.
Find more information online:
Assess How Serious the Problems Are
| Be concerned if reviewers had no major criticisms, but your application got an unfundable score. |
|
Read the summary statement carefully and analytically, keeping in mind that it is not an exhaustive critique.
If You Get Little Criticism
It's a paradox, but faint praise can be a worse sign than abundant criticism. You should be concerned if reviewers had no major criticisms of your application, but it got an unfundable score.
- Often this means reviewers were not excited about your idea. They may not state this explicitly, mostly out of politeness. Same for your program officer.
- Try to get honest feedback from coworkers or mentors, and don't shoot the messenger. It's better to find out at this stage than to keep trying with a doomed idea.
- If a dull topic was the problem, revising won't help. Start over with a new hypothesis.
If Reviewers Noted Many Fixable Problems
Surprisingly, it may be a good sign if reviewers pointed to lots of fixable problems. It often shows they are interested in the idea and are indicating it's worth revising.
See Know What a Summary Statement Means.
 |
Based on the analyses above, are the problems in your application fixable?
- Definitely fixable. Read Resubmission Timing below.
- Definitely not. Skip ahead to read these two pages and pick an approach: Option 3 and Option 4.
- Still not sure. Continue reading here for more analysis.
|
Common Fixable Problems
| You can correct many types of problems by revising the application. |
|
The following list shows problems you can fix by revising your application:
Problem: Poor writing.
Solution: Rewrite; get help with writing and editing.
Problem: Insufficient information, experimental details, or preliminary data.
Solution: Assess what's missing; add it to the Research Plan.
Problem: Significance not convincingly stated.
Solution: Beef up that section; show the importance to NIAID's mission, your area of science, and public health.
Problem: Research not shown to be feasible by the proposed staff.
Solution: Get consultants with the required expertise.
Problem: Insufficient discussion of obstacles and alternative approaches.
Solution: Describe what you'll do if you get negative results or an approach doesn't pan out. Include decision trees.
Problem: Reviewers are not interested in the subject.
Solution: Were they the appropriate reviewers? If not, request a different review group for your next submission. See Option 2: Revise and Resubmit to a Different Study Section. If yes, then this problem is not fixable -- see the next section.
Not Fixable or More Difficult Problems
| Some problems are very difficult or impossible to overcome. |
|
The following problems are either not fixable or are very difficult to correct:
If the problems are not fixable, you'll need to start over with a new idea. See Option 4: Look For Funding Outside NIH.
Pick a Strategy That Suits the Problem
| If you must revise more than 50 percent, create a new application. If less, prepare a resubmission. |
|
Once you've determined whether your problems are fixable, pick one of these four options:
- For fixable problems, choose one of the following:
- Revise the application and resubmit it to the same study section.
- Revise the application and resubmit it to a different study section.
- For nonfixable problems, choose one of the following:
- Create a new application.
- Look for funding outside NIH.
- If you responded to a request for applications and did not succeed, NIH allows you to submit the same application as a new investigator-initiated application.
- Find a new funding opportunity for investigator-initiated applications, such as the R01 parent announcement. Transfer your application information into the new Grant Application Package.
- Follow all the instructions for a new application. Don't refer to it as a resubmission anywhere.
- You can use the review comments to improve your application, but don't mark the changes as you would for a resubmission.
To gauge whether an application would be considered new or revised, use this rule of thumb: if you revise more than 50 percent, it's a new application. If less, follow the rules for a resubmission.
 |
Are you allowed to resubmit?
- No. Skip ahead to read these two pages and pick an approach: Option 3 and Option 4.
- Yes. Continue reading here.
|
 |
Did reviewers think your basic idea was interesting and important but found fixable problems?
|
 |
Do you have major reservations about the reviewers' understanding of your proposal?
|
 |
Did your application have bigger problems than those addressed in Option 1 or Option 2?
|
Option 1: Revise and Resubmit to the Same Study Section
If reviewers thought your basic idea was interesting and important but found fixable problems, the application is likely worth revising. Revising allows you to retain most of your original application, while addressing the reviewers' concerns.
Revising and resubmitting to the same study section is often advantageous. The study section looks at the application in the context of their critiques, so this approach is effective if you can readily answer their concerns.
This route is the most common one and works well when the points of contention are limited.
- Discuss each of the reviewers' points one by one.
- Show clearly in the text where you have made changes, for example, by using brackets, indents, or some other marker (not color because the application is photocopied).
- Include any new preliminary data, and strengthen the application where possible.
You can revise and resubmit your application only once.
 |
Will you use Option 1?
|
Option 2: Revise and Resubmit to a Different Study Section
Follow the advice for option 1, but request a change of study section if you have major reservations about the reviewers' understanding of your proposal. See Option 1: Revise and Resubmit to the Same Study Section for more on revising.
Get advice from mentors and your program officer. Then ask the following questions:
- Did your study section's interests match those of your application?
- Were the members comfortable with your methods?
If not, it may have been the wrong group. Here's what to do:
- Revise and strengthen the application.
- Look for a study section whose expertise best matches your topic and approach, and request the Center for Scientific Review to assign your application there.
- Suggest an alternative study section, but keep in mind that a special emphasis panel of ad hoc members may review your application.
- Frame your request in positive terms even if you believe there was a problem with a reviewer.
- For example, say that another study section has several people on it who are interested in your area and qualified to judge your work. Don't request reviewers by name or they will be disqualified!
- Spell out ideal reviewer competencies.
- State the reasons for the request, e.g., a lack of interest or differing philosophies.
- Request the study section change in your cover letter.
- Include any new preliminary data and strengthen the application where possible.
See Do You Need a Cover Letter? and Requesting a Study Section.
 |
Will you use Option 2?
|
Option 3: Create a New Application
Create a new application if your old application had bigger problems than those addressed in option 1 or 2 or if you've already sent an unsuccessful resubmission.
Though you may be able to reuse parts of the old application, you will need to significantly revise all sections of the Research Plan and other parts.
Make sure the following items are new, not just reworded:
- Title
- Hypothesis
- Specific Aims
- Abstract
For some other sections such as Background and Significance and Research Design and Methods, you may be able to reuse some of your previous text, but revise heavily.
Make sure any problems in the text you're keeping are fixable -- see Common Fixable Problems above. Your overhaul must include a new direction and approach. NIH expects the application to be substantially different in content and scope.
Going this route, reviewers will not see your summary statement from any previous review, so you get a fresh start.
- Be aware that NIH checks to make sure your application is sufficiently changed. Do not change just the title and make minor revisions.
- If your application ends up with the same study section, it's not a problem as long as you follow the guidance above.
- Be sure to include any new preliminary data, and strengthen the application where you can.
- See Option 2: Revise and Resubmit to a Different Study Section for help finding and requesting a study section.
- Talk with your program officer for more advice.
 |
Will you use Option 3?
- Yes. Follow the guidance above, and get more help from the NIH Grant Cycle.
- No or not sure. Continue reading here.
|
Option 4: Look For Funding Outside NIH
Explore all funding options.
- Be aware that you can send any application to NIH and to another organization simultaneously, although you will be able to accept only one award.
- Look into other funding sources.
 |
Will you use Option 4?
|
How to Revise and Resubmit
This is not advice: Once you are resubmitting, you're playing by new rules.
| Carefully address reviewers' comments point by point; make new text easy to distinguish. |
|
Your revised application must address all the concerns listed in your summary statement in an introduction and in the body of the Research Plan. Reviewers will make sure you discussed their comments and revised accordingly.
- Carefully address the comments point by point and make new text easy to distinguish.
- Remember -- there is no guarantee of success for several reasons:
- Reviewers are not wedded to the critiques.
- New reviewers may disagree with previous comments or raise new criticisms.
- Because a summary statement is not an exhaustive critique of your proposal, it may not list all problems reviewer may have. For more insights, read Summary Statements Have Their Limitations.
With those caveats in mind, take heart that many people get funded after revising. You must create an introduction and a cover letter for your resubmission. See Do You Need a Cover Letter?
NIH allows you to resubmit an unsolicited application submitted on January 25, 2009, and later only once with no time limit. You may resubmit an application sent before that date twice and must do so by January 7, 2011.
Include an Introduction
| Summarize all your additions, deletions, and changes to the old application. |
|
You must have an introduction in your Research Plan that summarizes all your additions, deletions, and changes to your old application.
- State how you responded to reviewers' comments and addressed the criticisms in the summary statement.
- Check the funding opportunity announcement for page limits.
- For an R01 application, your introduction page limit is three pages.
- Other grant types have different limits; for example, the R03 and R21 are limited to one page.
- Use the form section called Introduction to Application of the PHS 398 Research Plan form.
- For more instructions, see the Grant Application Guide for your Grant Application Package.
Resubmission Timing
Consider revising and resubmitting right away if your application scores above the payline, and its problems are fixable.
Many people start revising even before getting the summary statement because waiting may cause them to miss the next receipt date. (New investigators get summary statements earlier than others do.)
If you are on a list for possible selective pay or end-of-year funding, don't wait -- revise and resubmit.
Should You Start Revising Quickly?
- You could start revising before you get your summary statement if you have the following information:
- Insights into peer review from your program officer, who attended the review meeting and told you about the discussion.
- Promising new data or other improvements you want to include.
- Then after you get your summary statement, add to the revisions you've already made to address peer reviewer concerns. More on this above at Know What a Summary Statement Means.
When Not to Resubmit Early
- Always wait to resubmit until you have the strongest application possible -- better to wait for the next receipt date than send an application before you're ready.
- Sometimes, waiting to spend more time polishing your application is a better strategy than rushing to meet a receipt date, and the delay may have little impact on timing of an award.
- At the end of the fiscal year (June-July non-AIDS receipt date, September-October Council), you often have to wait several extra months before you get an award because the Institute does not yet have a budget for the following fiscal year.
- If you wait to submit for the October-November non-AIDS receipt date instead, you could lose just a month or two before you actually get an award.
- See the NIAID R01 Application to Award Timeline for more information.
Can a Resubmission Hurt You?
Usually a resubmission can't hurt you.
- Most resubmissions score higher than the initial application, though, of course, there's no guarantee. During fiscal years 1996 to 2006, more than 80 percent of resubmissions got better scores, and less than 5 percent got significantly worse scores.
- A resubmission that scores slightly lower probably won't affect the funding chances of an earlier application.
- If you've submitted two applications, NIAID can still fund the earlier one.
- eRA Commons will keep both versions of your application active.
- Related applications have the "MAA" (Multiple Active Application) flag in the eRA Commons; use the Status module.
- When one application is funded, NIH automatically withdraws the other.
- Keep in mind that if reviewers find major problems not detected in the initial application, your resubmission may score significantly lower.
NIH gives you only one resubmission, so you need a new strategy after that. Pick an approach: Option 3: Create a New Application and Option 4: Look For Funding Outside NIH.
Resubmission Tips
| Respond point by point to the reviewers' comments and suggestions even if you disagree. |
|
Here are some tips to help you succeed.
- Capitalize on your strengths and throw out or revise the parts reviewers felt were weak. Check again that your Specific Aims line up with your hypothesis.
- Respond point by point to the reviewers' comments and suggestions even if you disagree.
- If you disagree, explain why, and provide additional information if possible.
- Even better, change your proposal. For example, if a reviewer does not like an approach, propose a different one, even if you don't agree.
- If several years have elapsed since you submitted the application, the reviewers' comments may no longer be relevant.
- Resubmission caveat. Even if you revise following reviewers' comments, your priority score may not improve; for example, the study section may find new problems.
- Identify all changes. Use arrows, brackets, indents, or a new font. You can use Arial, Helvetica, Palatino Linotype, Georgia typeface, or a combination of the above. All fonts used should be black, 11 points or larger.
- If most of the text has changed, state that in the introduction rather than doing the above.
- Do not underline, shade changes, or use color.
- Avoid italicizing large blocks of text -- it's hard to read.
- Add new findings and your own changes. Though you must revise items mentioned in the summary statement, you aren't limited to those items.
- In the Preliminary Studies/Progress Report section, add any new findings you've gotten since the previous application.
- Don't hesitate to make other changes. Strengthen the application as much as you can.
Find more information online:
Help us improve our outreach to you by emailing deaweb@niaid.nih.gov. |