|
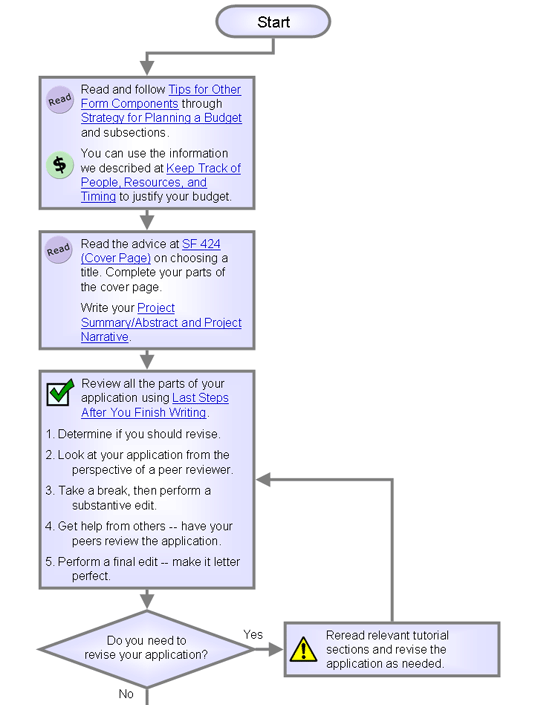 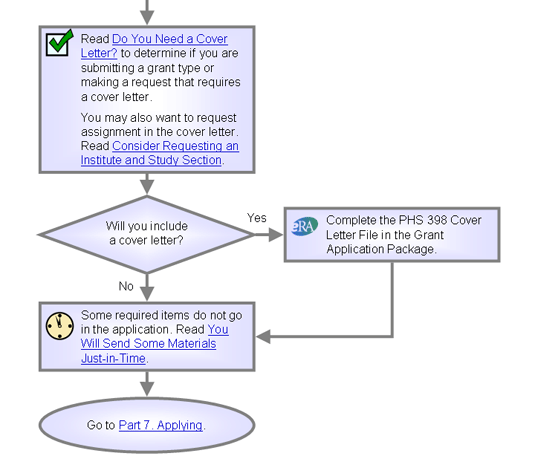
Table of Contents
Are You Ready for This Part?
Part 6. Other Application Sections gives you advice on sections of your application other than the Research Plan and helps you keep all the parts on target and consistent.
Before reading this page, be sure that you . . .
Tips for Other Form Components
| Check for consistency. Some information, such as key
personnel, resources,
and consortium, appears on more than one form. |
|
Congratulations!
You have completed the hardest part of your application: the Research Plan. Now, you're ready to tackle the rest of your R01 application.
Mandatory and "Optional" Components
Complete all mandatory components and any optional ones appropriate to your application. For the budget forms, "optional" means "pick the appropriate one."
Your funding opportunity announcement tells you which forms in the Grant Application Package are required; the Grant Application Guide explains how to fill out the forms.
 Our Advice: Make Sure Your Application Is Consistent Our Advice: Make Sure Your Application Is Consistent
Some information -- such as key
personnel, resources,
and consortium -- appears on more than one form. One of your objectives is to make sure all information on the forms is consistent with your Research Plan.
You can ensure consistency throughout by referring to the running tab we advised you to start when creating your Research Plan -- see Keep Track of People, Resources, and Timing.
- In the Research and Related Senior/Key Person Profile, list all key personnel and include a biosketch for each one.
- For a modular budget, list all personnel in the Personnel Justification
attachment of your PHS 398 Modular Budget form (see Create Your Budget).
- For a nonmodular budget, the Research and Related Budget states the total funds requested for each key person.
Other Project Information Form: Bibliography and References Cited
| Include all relevant publications but limit the number to fewer than about 100. |
|
List all the publications you've cited in your Research Plan and other parts of your application. There is no
page limit.
 Our advice. Your list should
probably have fewer than around 100 citations, but don't skimp on essentials because this section helps show your breadth of knowledge of your field. Our advice. Your list should
probably have fewer than around 100 citations, but don't skimp on essentials because this section helps show your breadth of knowledge of your field.
Use the Bibliography and References Cited attachment of the Research and Related Other Project Information form.
NIH accepts any standard citation style. Each citation must list the names of all authors
(do not use "et al.") in the same order as in the source publication plus the article title, name of the book or journal, volume number, page
number range, and year of publication.
Keep in mind that you have to follow NIH's public access policy when citing a paper that results from NIH funding. This requirement applies to new and renewal applications and progress reports.
Include a PubMed Central identification number in the citation. See the Public Access of Publications SOP for more information, including what to do if a PMC ID is not ready.
- Sample citation using the PubMed Central number:
- Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD, Smith DM. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. J Virol. 2005 79(3):1734-1742. PMCID: PMC544119.
Note: the PubMed Central ID and PubMed ID numbers are different. If you have the PubMed ID, you can use the National Library of Medicine's PM ID: PMC ID Converter to find out the PMC ID or vice versa.
See Reference
Publications for more tips on adding references to your application.
Other Project Information Form: Facilities, Resources, and Equipment
| Convince reviewers you
have the resources needed to conduct the research. |
|
 Our Advice: Make a Case for Your Resources Our Advice: Make a Case for Your Resources
In
this section of your application, convince reviewers you
have the equipment, space, staff, and facilities to conduct the research. Show them that your institution provides the support you need and does not demand excessive amounts
of your time.
If your science is elegant but you don't have the resources
to carry it out, reviewers will not be at all impressed.
Facilities and Other Resources Attachment
- State essential resources such as the following.
- Animal facilities.
- If you are at an AAALAC-accredited
institution, you don't need to detail basic items such as the number of animal cages.
- If you are not at an AAALAC-accredited institution,
spell it all out.
- Biocontainment resources
for research involving select agents.
- Make sure the sites you list match those in the Research and Related Project/Performance Site Locations form.
- If your research involves more than one site, state which
facilities are completing which parts of the project, and describe key resources for each one.
Equipment Attachment
- List major items of equipment available to you; give their location and capabilities.
List even basic items if you are at a research
institution that gets little NIH funding.
Use the Research and Related Other Project Information form in the Grant Application Package. Also see Additional Performance Sites (if more than eight performance sites).
Senior/Key Person Profile Form: Prepare the Biographical Sketches
| Convince reviewers your team has the experience to conduct the research. |
|
Reviewers look carefully to see whether the PI and others have enough experience with the techniques to execute the Research Plan.
Make sure this section is consistent with the running tab we advised you to start when creating your Research Plan -- see Keep Track of People, Resources, and Timing.
You will create a biosketch for all people who play a substantive role in the project even if they are not paid a salary from the grant, including consultants and technical staff.
Even if you know someone will be gone by the time the application is funded, keep that person on the personnel list. Reviewers understand that
people move and assume you will find replacements with similar skills.
Though four pages are standard, you may submit fewer. Use the Biographical Sketch Sample as an example of the format follow.
Fill out a copy of the Biographical Sketch form from the NIH Web site, and attach it to the Research and Related Senior/Key Person Profile form. Be sure to enter your Commons ID as the "Credential, e.g., agency login."
- Do not attach current and pending support, a.k.a. other support, unless (rarely) your FOA instructs you to do so. We will request this just-in-time if we plan to fund your application.
- Do not include pending support.
- Read the Grant Application Guide for more instructions.
Use the Research and Related Other Project Information form in the Grant Application Package.
Find more information online:
Strategy for Planning a Budget
| Don't request more money than your research requires. |
|
As you design your experiments, you will continually revise and keep track of the size of your budget.
Expect to spend time and effort writing a thorough justification
of your budget. The more detail you include to justify it, the better -- weak budget justifications are a big problem for many applications.
Make sure you make this section consistent with the running tab we advised you to start when creating your Research Plan -- see Keep Track of People, Resources, and Timing.
For guidance on how much money and time to request, read Think About Scope, Amount, and Effort and then the advice below.
 Our Advice: Know How Much Money to Request Our Advice: Know How Much Money to Request
- Ask for only enough money to do the work.
- Reviewers look for reasonable costs and will judge whether
your request is justified by your Specific Aims and
methods.
- Reviewers will read the percent effort you've listed
for each key person and will judge whether the figures are in sync
with their expectations based on the research you proposed.
- Significant over- or under-estimating suggests you don't understand
the scope of the work.
- As a rule of thumb for calculating your costs, figure
salaries will be 60 to 80 percent of the total request.
- Make sure the PI's salary
takes into account the mandatory cap, which changes every fiscal
year. Check our Paylines and Budget page, the NIH Guide, or your business office for the latest figure.
- Do not ask for anything that can appear to be extravagant, such as a lot of travel.
- If you are requesting $500,000 or
more in direct
costs in any grant year, you will need NIAID's approval before you may submit an investigator-initiated application; document the approval in your cover letter. For details, see the Big Grants SOP.
Your business office can inform you of your institution's facilities and administrative cost (indirect cost) rate.
To decide if you need to use a modular or detailed budget, read the next section.
Use the Research and Related Budget components and PHS 398 Modular Budget forms in the Grant Application Package.
Find more information online:
Develop a Modular Budget
| Reviewers will judge how well your funding request matches the project's scope as a gauge of your competence. |
|
If your budget is less than $250,000 and you are from a domestic institution, prepare a modular budget that requests funding in $25,000 increments.
If your budget is more than $250,000 or you are from a foreign institution (or both), see Detailed Budgets below.
The following applications must be modular when budgets are less than $250,000, unless stated otherwise in a request for applications (RFA) or program announcement (PA):
- Research project grant (R01)
- Small research grant (R03)
- Exploratory/developmental grant (R21)
- Academic research enhancement award (R15)
- Clinical Trial Planning Grant (R34)
Because modular budgets have no increases for inflation for future years, you'll have to plan the entire budget -- everything you'll need -- at the outset.
 Our advice. If you've never done this before, get help. Reviewers will view the correlation of your funding request with the project's scope as a gauge of your competence. Our advice. If you've never done this before, get help. Reviewers will view the correlation of your funding request with the project's scope as a gauge of your competence.
Using a Modular Budget
Most new investigators "go modular" -- about 80 percent of them in FY 2006.
Always create a detailed
budget if you are responding to a request
for applications or program
announcement that requires one or if you
are from a foreign institution.
Generally people request the same number of modules each year, except for special needs such as equipment.
If you vary the number of modules, justify it in the Additional
Narrative Justification attachment of the PHS
398 Modular Budget form.
For consortium information, see Where to Add Consortium and Contractual Information. Read the Grant Application Guide for additional instructions.
Find more information online:
Create Your Budget
| Don't request funds for equipment or resources you've already indicated are available to you. |
|
 Our advice. If
your budget is ballooning out of scale for your grant
type or career stage, consider cutting back experiments or Specific Aims. Our advice. If
your budget is ballooning out of scale for your grant
type or career stage, consider cutting back experiments or Specific Aims.
Read the preceding pages to determine whether to prepare a modular
or
detailed budget.
 |
Are you requesting less than $250,000?
- No. Skip ahead to Detailed Budgets.
- Yes. Continue reading about modular budgets.
|
Modular Budgets
After
you've determined your budget in increments of $25,000, fill in the PHS 398 Modular Budget form with your budget request for each year and the total. You can find detailed instructions in the Completing PHS 398 Components section of the Grant Application Guide.
On the last page of the form, find and complete the attachments for three justifications: Personnel, Consortium, and Additional Narrative.
- Personnel Justification
Attachment
- List all personnel (not just key personnel), their roles, and number of person months to be devoted to the project.
- Briefly describe responsibilities
in enough detail to justify a person's
level of effort. Regular research projects (e.g., R01s) do not have effort
requirements, but career development, training, and small business awards often do.
- Do not give salary information. Use the NIH salary limit for the PI and graduate students to estimate costs. Go to Salaries and Stipends questions and answers.
- Include key personnel even if you know they will be gone by the time the application is funded.
- Consortium Justification Attachment
- Additional Narrative Justification Attachment
- Number of modules. Write a justification if the number of modules requested varies from year to year.
 Our Advice: Steer Clear of Asking for Money for Equipment at Hand Our Advice: Steer Clear of Asking for Money for Equipment at Hand
- Equipment. In general, don't request funds for equipment or resources you've listed as available in the attachments to your Research and Related Other Project Information form.
- Reviewers will check. They'll delete the funds, and your credibility will suffer.
- Avoid asking for expensive equipment
unless you absolutely need it. If you do need the equipment,
justify it well.
- If you'll need to purchase equipment costing more than $25,000, create a separate module. Make it a one-time request; do not add it to your base amount.
- Is your equipment old? If so, consider adding funds to replace aging equipment.
Detailed Budgets
Prepare a detailed budget if you are requesting more than $250,000 or applying from a foreign institution.
- Use the Research and Related Budget component forms.
- Do not request a salary over the annual cap. See our PI Salary Cap and Stipends information.
- Make sure your direct costs and facilities and administrative costs (F&A, aka, indirect costs) are consistent on all budget pages.
- Use whole numbers for person months for percent effort and dollars for costs -- round calculations to the nearest dollar. F&A rates do not need to be whole numbers.
- Read the instructions in the Grant Application Guide.
SF 424 (Cover Page)
| Your title and Abstract affect how NIH assigns your application and reports your research to Congress. |
|
You will complete parts of the SF
424 (Cover Page) form, and your business office will prepare
other parts.
Your business
office must sign before you submit your application. As PI, you do not sign it. Before each submission,
you provide your institution a unique signature
assurance that it will keep on file.
 Our Advice: Contact Your Business Office Early; Choose the Right Title Our Advice: Contact Your Business Office Early; Choose the Right Title
Visit the people in your business office early to see what information they need and how much
time they'll need to review, add required information to, and
sign your application.
Writing your title. Your title and Abstract affect how NIH will
assign your application and report your research dollars to Congress.
We recommend you finalize the title and
Abstract last. NIH referral officers depend on your Abstract and title
to assign your application to a
study section and institute.
Make your title specific and detailed.
- Your title must be unique. Make sure it differs from that of
your other Public
Health Service applications or awards.
- Make sure it has suitable keywords so NIH referral staff
will assign your
application to the appropriate institute and study
section, and NIH computer systems can retrieve your
grant properly.
- For a resubmission, you usually keep the same title. See Part 11b. Not Funded, Reapply.
- For a renewal,
you usually keep the same title, but it may be advantageous to
change it if your research is going in a new direction or has
a significant change in scope.
Read more in the Part 12. Renewal Application.
- If your application is a revision (competing supplement),
keep the same title.
- For an explanation of the terms above, see
the NIAID Glossary of Funding
and Policy Terms and Acronyms:
If you're a new investigator. Check "yes" in the box on
the PHS
398 Cover Page Supplement form. For more on qualifying, see Are You "New"? in the New Investigator
Guide to NIH Funding tutorial.
If you propose human embryonic stem cell research. Enter the Human Embryonic Stem Cell Registry number in the stem cell section in the PHS 398 Cover Page Supplement or state that you will use one from the NIH registry.
Read more at NIH's Stem Cell Information.
Project Summary/Abstract and Project
Narrative
| Your Abstract
should state your hypothesis, Specific Aims, design and methods, and long-term objectives. |
|
After you have finished writing the other parts of your application,
write your Project
Summary/Abstract and Project
Narrative using the Research
and Related Other Project Information form.
 Our Advice: Be Brief, Pick Proper Keywords, and Use Lay Language Our Advice: Be Brief, Pick Proper Keywords, and Use Lay Language
Keywords you use can affect the assignments NIH's Center for Scientific Review will give you to an institute and study
section or integrated
review group.
Project Summary/Abstract
- Write a succinct summary of your project that both a scientist
and a lay person can understand. Expect all the peer
reviewers to read your Abstract.
- Describe the importance of your research to your field and
relevance to NIAID's mission: to better understand, treat, and
prevent infectious, immunologic, and allergic diseases.
- State your hypothesis, Specific Aims, design and methods, and long-term objectives.
- If your application is funded, your application's title and abstract become a public text document in the NIH CRISP database. Don't
include confidential or proprietary information.
- Don't include graphs or other images.
- Make sure your Abstract has appropriate keywords so NIH referral
staff can assign your
application and NIH computer systems can retrieve your
grant properly.
Project Narrative
- In lay language, describe your project's potential to improve
public health in two or three sentences.
- Don't include graphs or other images.
Last Steps After You Finish Writing
| Take a fresh look at your application with a peer reviewer's perspective. |
|
 Our Advice: Allow Time For Checks, Get Help to Make It Perfect Our Advice: Allow Time For Checks, Get Help to Make It Perfect
Now that you have drafted all the application pieces, check that the main parts are on target and in sync with each other.
Allow time to do this because revisions in one section may trigger other changes. For example, if your budget is out of scope, you may need to adjust your Specific Aims, which will change other parts of your application.
1. Ask these questions to determine if you should revise.
2. Look at your application from the perspective of a peer reviewer.
3. Take a break, then perform a substantive edit.
- After you've finished writing, put the application aside for a few days. Then do the following.
- Check for consistency, organization, and other items.
- Is the material well organized with all elements easy to find?
- Did you avoid including confidential information in the title or abstract? Did you mark any other confidential information to remind reviewers?
- Did you reference the latest publications and key findings in your field? Did you support all facts with citations?
- Does the writing use topic sentences that clearly state each main point?
- Is the application visually appealing, with white space and elements such as bullets to break up and organize the text?
- Did you cross check all data and information for consistency? Did you check information that appears on more than one application form, such as key personnel, resource, consortium, and budget?
4. Get help from others -- have your peers review the application.
- Get opinions from colleagues in your field who are successful grant writers, preferably people who have been members of NIH study sections.
- Ask for opinions on the writing and presentation as well as the content.
- The more critical they are, the better. You want to know the problems before you send in your application rather than learn about them from the NIH peer review.
- Also have nonexperts in the field read the application to make sure it's clear and understandable. Give your reviewers at least two weeks. Then, allow yourself a full week to incorporate their suggestions.
5. Perform a final edit -- make it letter perfect.
- After you're happy with the content, perform the last step: a final edit.
- Enlist friends to help.
- You and they will probably find lots of errors you overlooked before. Check for consistency, typos, and grammatical mistakes, omitted information, and errors in figures and tables.
- Make sure your work is letter perfect.
- Edit out redundant words and phrases; make your writing concise and informative.
- Proofread several times on different occasions, and have others proofread as well, including nonscientists with strong English skills.
- If you cannot meet the application deadline comfortably, consider delaying to the next receipt date.
Find more information at Write, Edit, and Proof Like a Pro.
 |
Did you review the list above to make sure you have the strongest possible application? Did you conduct your own peer review?
- No. Reread the section above and make improvements, then continue reading.
- Yes. Continue reading.
|
Do You Need a Cover Letter?
We recommend that you include a cover letter for all applications, and you must have one for the following:
- Resubmissions.
- Applications that require NIAID approval, for example:
- To request $500,000 or more in direct costs for any one year. Get more detailed information in the Big Grants SOP.
- To submit an Investigator-Initiated Clinical Trial Planning and Implementation Grant (R34).
- Conference grant applications. Get more detailed information in the Conference Awards SOP.
- Genome-wide association studies or those that plan to access GWAS data in the NIH repository. To read more on that now, refer to What Resources Do You Need to Share?
- Late applications. Explain the reason for the delay. For the policy, see the Late Applications SOP.
- Corrected applications. Be sure you include a complete cover letter.
Key Points for Any Cover Letter
Start with a brief description and state the title of your application. Use the format NIH gives you in the Grant Application Guide.
| List people who should not review your application. |
|
 Our Advice: Know What a Cover Letter Can Do for You Our Advice: Know What a Cover Letter Can Do for You
A cover letter is extremely helpful to accomplish the following:
- Exclude people. List people who should not review your application, and give a reason, e.g., you have a long-standing scientific disagreement.
- State your objections but focus on the positive; for example, list skills needed to review the application.
- Make sure your competitors are not your reviewers!
- Request assignment from the Center for Scientific Review to a study section and institute. More on that on the next page, Consider Requesting an Institute and Study Section.
- List expertise needed to understand and review your application, whether you request a study section or not.
- Note approval letters
- State that you have attached the Institute approval letter for a grant requesting $500,000 or more in direct costs for any year. Get more detailed information in the Big Grants SOP.
- State that you have attached our approval for a conference grant (R13 or U13). See the Conference Awards SOP.
- State that you have attached our approval for an R34. Go to Investigator-Initiated Clinical Trials Resources.
- State that you are an appointed (not ad hoc) member of an NIH study section submitting at a nonstandard time.
- Members of NIH study sections may submit at any time an R01, Exploratory/developmental grant (R21), or Clinical Trial Planning Grant (R34) application that is due on a standard receipt date (not, for example, applications responding to requests for applications).
- If you submit at a nonstandard time, you cannot request assignment to a study section.
- Include the name of the study section you're serving on and your period of service.
- Point out RFAs and PAs. State the title if you're responding to an initiative.
- Note special areas. Note the involvement of human subjects, select agents, genome-wide association studies or study data, or other areas with special requirements.
- Note a subaward to be active only some of the grant's years.
Don't confuse the PHS 398 Cover Letter File with the mandatory PHS 398 Cover Page Supplement form. Use the latter to identify yourself as a new investigator -- see our definition at Are You "New"? to determine if you are one.
Read the Grant Application Guide for formatting and other requirements.
Use the PHS 398 Cover Letter File in the Grant Application Package.
 |
Are you submitting a grant type or making a request that requires a cover letter?
- Yes. Create a cover letter following the advice above and instructions in your Grant Application Guide, then continue reading here.
- No. Continue reading.
|
Consider Requesting an Institute and Study Section
| Consider requesting an assignment -- even if you're a new investigator! |
|
 Our Advice: Tell NIH Where You Want Your Application to Go Our Advice: Tell NIH Where You Want Your Application to Go
As you finalize your application, think about requesting assignment to an institute and study section or integrated review group in your cover letter.
For advice, talk to a program officer or scientific review officer. Use the PHS 398 Cover Letter File in the Grant Application Package.
Look at the CSR Study Section Roster Index for study sections likely to review your application so you can check the background and expertise of members and determine which committee would be the best one.
In addition to its referral staff, CSR uses knowledge management techniques to assign applications. To enable these systems to read your request, use the following format:
- Put each request on a separate line.
- Put positive and negative requests on separate lines.
- Include name of institute and scientific review group followed by a dash and the acronym.
- Explain each request in a separate paragraph.
- Example
- Institutes/Centers
- National Institute of Allergy and Infectious Diseases – NIAID
- National Cancer Institute – NCI
- Scientific Review Groups
- AIDS-Associated Opportunistic Infections and Cancer Study Section – AOIC
- AIDS Immunology and Pathogenesis Study Section – AIP
- Please do not assign this application to the following:
- Scientific Review Groups
- AIDS Molecular and Cellular Biology Study Section – AMCB
- The reasons for this request are: (short explanation of your requests).
If appropriate, read If I respond to an NIAID program announcement, can I request a study section? in the Peer Review of Applications -- CSR questions and answers.
If you do not request assignments, NIH will do this for you using its referral criteria.
Learn more about the assignment process in Applications Are Assigned to an Institute and Integrated Review Group.
Find more information online:
Requesting an Institute
Contact the program
officer for your area of science:
|
|
Though you do not need to identify an institute, having your application assigned to an institute that is enthusiastic about your research may improve your funding chances.
If you haven't already done so, talk to a program
officer and do some research on the Web into the scientific areas
each institute funds. You can see NIAID's at Research by Topic.
 Our Advice: Check Around to See Where Your Application Might Fit Our Advice: Check Around to See Where Your Application Might Fit
- Contact an NIAID program officer to determine the following:
- Does the application fit any of our funding opportunity announcements or other areas of interest?
- Do we use your mechanism of interest in your topic area?
- Do we have Institute-specific criteria?
- If not interested in an application, some institutes will skip it for funding even if it ranks within the payline -- NIAID does not.
Is the Institute Interested?
Always talk to a program officer before requesting assignment. Read how to gauge an institute's interest at Where Does Your Research Belong?
If you feel NIAID would be the best home for your application, request assignment here in your cover letter. Use the PHS 398 Cover Letter File in the Grant Application Package.
If you do not make this request, CSR will assign your application using keywords in your title, Project Summary/Abstract, Background and Significance, and Specific Aims.
Should You Look Into Paylines?
No. Before applying, institute paylines are not useful. By the time you get funded, a payline can change and so is not a helpful gauge of funding probability.
Plus, Institute paylines can be deceiving. NIAID sets conservative paylines each fiscal year. At the end of the fiscal year, we often fund some deferred grants, and paylines go up.
Paylines are useful when you are about to get funded. Then you can check to see if your application scored within the payline and plan your strategy if it did not.
For more information, see Paylines and Budget Page Changes Throughout the Year and NIAID R01 Application to Award Timeline.
Find our current paylines on our Paylines and Budget page. Many other institutes also publish their paylines on their Web sites. Go to Institutes, Centers, and Offices.
 |
Do you want to request assignment?
|
 |
Is NIAID interested in your application?
|
Requesting a Study Section
| Get your application assigned to a study section where some members know your work and potential. |
|
By requesting assignment to a study section, you help ensure that the right people review your
application, and you exclude your competitors. Less frequently, applicants request an integrated review group to choose a group of study sections.
 Our Advice: Get to Know Study Sections Our Advice: Get to Know Study Sections
Gathering the information to make an informed request takes a bit of work, but many investigators feel it's worthwhile. Look up study section members at CSR Study Section Roster Index, and cite their work if relevant. To see science areas, go to CSR's Scientific
Areas of Integrated Review Groups.
Try to get your application assigned to a study section where some members know your or a collaborator's work and potential.
How to Request a Study Section
Follow these guidelines:
- Find a group you feel would appreciate your ideas.
- Make sure the study section is truly the one you want.
- Be aware that NIH may reconfigure study sections.
- Seek familiar
names.
- If the area seems right but you don't recognize anyone,
read some papers by the members.
- If
the members seem to be working in very different areas or are likely to have
competing world views, go elsewhere.
For example, if your approach
is functional genomics, you don't want to be reviewed by a study
section populated by cellular and molecular biologists.
- One caveat: it's not easy to tell who will review the application because CSR uses many ad hoc reviewers.
- Get advice from people in your institution.
- Contact scientific review officers for help determining which study section is appropriate.
- Frame your request in positive terms. Say that a study section has several people who are interested in your area and qualified to judge your work.
- Never suggest reviewers. If you do, they are immediately disqualified!
Example of an Acceptable
and Unacceptable Request
| Write your request with a clear justification. |
|
You could just state which study section you want, but it helps to explain the reason that study section would be a good match.
Write only a sentence or two -- CSR does not want more -- describing the areas of expertise needed to review the application. Example:
- The focus of reviewers on study section X seems
more on structural biology of molecules of immunologic importance.
Since my application proposes to develop new antibodies for Phase
I human studies, the clinical perspective of reviewers on study section Y is critical to appreciate the approaches
I have taken.
Use the PHS 398 Cover Letter File in the Grant Application Package.
Find more information online:
 |
Can you find a study section or IRG you feel would appreciate your ideas? Do you have competitors you want to exclude?
- Yes. Request assignment and mention the competitors in your cover letter. Read Do You Need a Cover Letter? above, then continue reading here.
- No. Contact the scientific review officer for
advice and continue
reading.
|
You Will Send Some Materials Just-in-Time
| Do not submit other support information with your application. |
|
Some required items do not go in the application. Instead, you send them just-in-time when your application is within a range of possible funding.
NIH uses just-in-time for other support information and several items for human subjects and vertebrate animal research. Find details in Prepare Your Just-in-Time Information and the Just-in-Time questions and answers.
 Our Advice: Prepare Your Just-in-Time Information Early Our Advice: Prepare Your Just-in-Time Information Early
Have your information ready well before we make the award. You may want to start preparing when writing the application or just after, so it will be ready when we ask for it.
Do not submit other support (called current and pending support in the Grant Application Package) with your application. If you do, NIH may delay processing it or return it to you without review.
Don't confuse research support with other support. Though
they sound similar, these parts of the application are very different.
- Research support highlights
scientific accomplishments for each person.
- It is a section in the biosketch attachment in the Research and Related Senior/Key Person Profile form.
- Reviewers read this section to assess the "investigator" initial peer review criterion.
- Other
support enables NIH to make sure research or resources you are requesting have not already been paid for.
Help us improve our outreach to you by emailing deaweb@niaid.nih.gov. |
 Our advice. If you've never done this before, get help. Reviewers will view the correlation of your funding request with the project's scope as a gauge of your competence.
Our advice. If you've never done this before, get help. Reviewers will view the correlation of your funding request with the project's scope as a gauge of your competence. Our advice. If
your budget is ballooning out of scale for your grant
type or career stage, consider cutting back experiments or Specific Aims.
Our advice. If
your budget is ballooning out of scale for your grant
type or career stage, consider cutting back experiments or Specific Aims.
 Our Advice: Steer Clear of Asking for Money for Equipment at Hand
Our Advice: Steer Clear of Asking for Money for Equipment at Hand  Our Advice: Contact Your Business Office Early; Choose the Right Title
Our Advice: Contact Your Business Office Early; Choose the Right Title Our Advice: Be Brief, Pick Proper Keywords, and Use Lay Language
Our Advice: Be Brief, Pick Proper Keywords, and Use Lay Language  Our Advice: Allow Time For Checks, Get Help to Make It Perfect
Our Advice: Allow Time For Checks, Get Help to Make It Perfect 
 Our Advice: Know What a Cover Letter Can Do for You
Our Advice: Know What a Cover Letter Can Do for You 
 Our Advice: Tell NIH Where You Want Your Application to Go
Our Advice: Tell NIH Where You Want Your Application to Go  Our Advice: Check Around to See Where Your Application Might Fit
Our Advice: Check Around to See Where Your Application Might Fit 

 Our Advice: Get to Know Study Sections
Our Advice: Get to Know Study Sections 
 Our Advice: Prepare Your Just-in-Time Information Early
Our Advice: Prepare Your Just-in-Time Information Early 