
NTP Study Reports

NTP Study Reports
Home » Study Results & Research Projects » NTP Study Reports » All Long-Term Reports » Abstract for TR-410 - Naphthalene
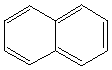
| Chemical Formula: C10H8 | - | 3D Structure* |
|---|---|---|
| *To view structure, download free Chemscape Chime Plug-in | ||
Naphthalene, a white, crystalline powder, is used as a moth repellent and in the manufacture of phthalic and anthranilic acids, naphthylamines, and synthetic resins. The 2-year studies were conducted by exposing groups of male and female B6C3F1 mice to naphthalene (>99% pure) vapor for 6 hours daily, 5 days per week, for 104 weeks. Genetic toxicology studies were conducted in Salmonella typhimurium and Chinese hamster ovary cells.
Groups of male and female mice were exposed to atmospheres containing 0 (75 mice per group), 10 (75 mice per group), or 30 ppm (150 mice per group) naphthalene. Mice from each group were included for 14-day hematology evaluations (male: 0 ppm, 5 animals; 10 ppm, 4; 30 ppm, 10; female: 0 ppm, 4; 10 ppm, 5; 30 ppm, 10). Mean body weights of exposed mice were slightly lower than those of controls throughout the studies. Survival of male control mice was significantly less than that of exposed mice; the lower survival was the result of wound trauma and secondary infections related to fighting among the group-housed mice (0 ppm, 26/70, 37%; 10 ppm, 52/69, 75%; 30 ppm, 118/133, 89%). Survival of exposed female mice was similar to that of controls (59/69, 86%; 57/65, 88%; 102/135, 76%).
No increase in tumor incidence related to naphthalene administration was observed in male mice. In females, the incidence of pulmonary alveolar/bronchiolar adenomas was significantly greater in the high-dose group than in the controls (5/69, 7%; 2/65, 3%; 28/135, 21%). One other high-dose female had an alveolar/bronchiolar carcinoma. The combined incidence of alveolar/ bronchiolar adenomas and carcinomas in the high-dose females was above those for control female B6C3F1 mice from NTP feed, water, and inhalation studies (91/1,166, 7.8%, range 0%-16%). These lung tumors were attributed to naphthalene exposure.
Nonneoplastic lesions attributed to naphthalene exposure were observed in the nose and lungs of mice of both sexes. In the nose, naphthalene exposure was associated with an increase in the incidence and severity of chronic inflammation, metaplasia of the olfactory epithelium, and hyperplasia of respiratory epithelium. Chronic inflammation in the lung was associated with chemical exposure.
Naphthalene was negative for the induction of gene mutations in Salmonella typhimurium strains TA100, TA1535, TA1537, and TA98 with and without exogenous metabolic activation (S9). In cytogenetic tests with Chinese hamster ovary cells, naphthalene induced sister chromatid exchanges with and without S9 activation. Exposure to naphthalene induced a significant increase in chromosomal aberrations in Chinese hamster ovary cells in the presence of S9.
Under the conditions of these 2-year inhalation studies, there was no evidence of carcinogenic activity of naphthalene in male B6C3F1 mice exposed to 10 or 30 ppm. There was some evidence of carcinogenic activity of naphthalene in female B6C3F1 mice, based on increased incidences of pulmonary alveolar/ bronchiolar adenomas.
In both male and female mice, naphthalene caused increased incidences and severity of chronic inflammation, metaplasia of the olfactory epithelium, and hyperplasia of the respiratory epithelium in the nose and chronic inflammation in the lungs.
Synonyms: Naphthalin, Naphthene, Tar Camphor
Report Date: April 1992
Pathology Tables, Survival and Growth Curves from NTP 2-year Studies
Target Organs & Incidences from 2-year Studies
You may link to the full technical report in pdf format ( Note: A print ready copy of the document is presented in Portable Document Format (pdf) which requires the Acrobat Reader plug-in -- download a free copy of the reader.)
Web page last updated on October 11, 2007
The National Institute of Environmental Health Sciences is one of the National Institutes of Health within the U.S. Department of Health and Human Services. The National Toxicology Program is headquartered on the NIEHS campus in Research Triangle Park, NC.