

Interstitial Cystitis / Painful Bladder Syndrome
On this page:
What is IC/PBS?
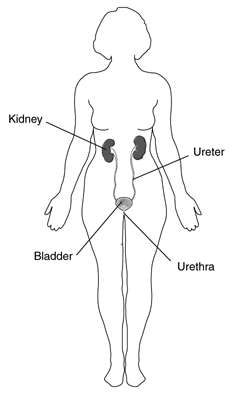 Interstitial cystitis (IC) is a condition that results in recurring discomfort or pain in the bladder and the surrounding pelvic region. The symptoms vary from case to case and even in the same individual. People may experience mild discomfort, pressure, tenderness, or intense pain in the bladder and pelvic area. Symptoms may include an urgent need to urinate, a frequent need to urinate, or a combination of these symptoms. Pain may change in intensity as the bladder fills with urine or as it empties. Women’s symptoms often get worse during menstruation. They may sometimes experience pain during vaginal intercourse.
Interstitial cystitis (IC) is a condition that results in recurring discomfort or pain in the bladder and the surrounding pelvic region. The symptoms vary from case to case and even in the same individual. People may experience mild discomfort, pressure, tenderness, or intense pain in the bladder and pelvic area. Symptoms may include an urgent need to urinate, a frequent need to urinate, or a combination of these symptoms. Pain may change in intensity as the bladder fills with urine or as it empties. Women’s symptoms often get worse during menstruation. They may sometimes experience pain during vaginal intercourse.
Because IC varies so much in symptoms and severity, most researchers believe it is not one, but several diseases. In recent years, scientists have started to use the term painful bladder syndrome (PBS) to describe cases with painful urinary symptoms that may not meet the strictest definition of IC. The term IC/PBS includes all cases of urinary pain that can’t be attributed to other causes, such as infection or urinary stones. The term interstitial cystitis, or IC, is used alone when describing cases that meet all of the IC criteria established by the National Institute of Diabetes and Digestive and Kidney Diseases.
In IC/PBS, the bladder wall may be irritated and become scarred or stiff. Glomerulations用inpoint bleeding caused by recurrent irritation熔ften appear on the bladder wall. Hunner’s ulcers are present in 10 percent of people with IC. Some people with IC/PBS find that their bladder cannot hold much urine, which increases the frequency of urination. Frequency, however, is not always specifically related to bladder size; many people with severe frequency have normal bladder capacity. People with severe cases of IC/PBS may urinate as many as 60 times a day, including frequent nighttime urination, also called nocturia.
IC/PBS is far more common in women than in men. Of the estimated 1.3 million Americans with IC, more than 1. million are women.1
1Clemens JQ, Joyce GF, Wise M, Payne CK. Interstitial cystitis and painful bladder syndrome. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: U.S. Government Printing Office; 2007. NIH publication 07–5112: 123–154.
[Top]
What causes IC/PBS?
Some of the symptoms of IC/PBS resemble those of bacterial infection, but medical tests reveal no organisms in the urine of people with IC/PBS. Furthermore, people with IC/PBS do not respond to antibiotic therapy. Researchers are working to understand
the causes of IC/PBS and to find effective treatments.
In recent years, researchers have isolated a substance found almost exclusively in the urine of people with IC. They have named the substance antiproliferative factor, or APF, because it appears to block the normal
growth of the cells that line the inside wall of the bladder. Researchers anticipate that learning more about APF will lead to a greater understanding of the causes of IC and to possible treatments.
Many women with IC/PBS have other conditions such as irritable bowel syndrome and fibromyalgia. Scientists believe IC/PBS may be a bladder manifestation of a more general condition that causes inflammation in various organs and parts of the body.
Researchers are beginning to explore the possibility that heredity may play a part in some forms of IC. In a few cases, IC has affected a mother and a daughter or two sisters, but it does not commonly run in families.
[Top]
How is IC/PBS diagnosed?
Because symptoms are similar to those of other disorders of the bladder and there is no definitive test to identify IC/PBS, doctors must rule out other treatable conditions before considering a diagnosis of IC/PBS. The most common of these diseases in both sexes are urinary tract infections and bladder cancer. In men, common diseases include chronic prostatitis or chronic pelvic pain syndrome. IC/PBS is not associated with any increased risk of developing cancer.
The diagnosis of IC/PBS in the general population is based on the
- presence of pain related to the bladder, usually accompanied by frequency and urgency
- absence of other diseases that could cause the symptoms
Diagnostic tests that help rule out other diseases include urinalysis, urine culture, cystoscopy, biopsy of the bladder wall, distention of the bladder under anesthesia, urine cytology, and laboratory examination of prostate secretions.
Urinalysis and Urine Culture
Examining urine with a microscope and culturing the urine can detect and identify the primary organisms that are known to infect the urinary tract and that may cause symptoms similar to IC/PBS. A urine sample is obtained either by catheterization or by the clean catch method. For a clean catch, the patient washes the genital area before collecting urine midstream in a sterile container. White and red blood cells and bacteria in the urine may indicate an infection of the urinary tract, which can be treated with an antibiotic. If urine is sterile for weeks or months while symptoms persist, the doctor may consider a diagnosis of IC/PBS.
Culture of Prostate Secretions
Although not commonly done, in men, the doctor might obtain prostatic fluid and examine it for signs of a prostate infection, which can then be treated with antibiotics.
Cystoscopy under Anesthesia with Bladder Distention
The doctor may perform a cystoscopic examination in order to rule out bladder cancer. During cystoscopy, the doctor uses a cystoscope預n instrument made of a hollow tube about the diameter of a drinking
straw with several lenses and a light—to see inside the bladder and urethra. The doctor might also distend or stretch the bladder to its capacity by filling it with a liquid or gas. Because bladder distention is painful for people with IC/PBS, they must be given some form of anesthesia for the procedure.
Biopsy
A biopsy is a tissue sample that can be examined with a microscope. Tissue samples of the bladder and urethra may be removed during a cystoscopy. A biopsy helps rule out bladder cancer.
Future Diagnostic Tools
Researchers are investigating and validating some promising biomarkers such as APF, some cytokines, and other growth factors. These might provide more reliable diagnostic markers for IC and lead to more focused treatment for the disease.
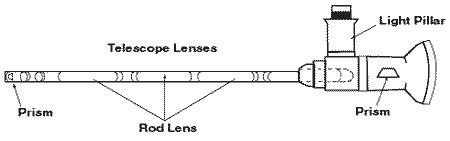
Cystoscope.
[Top]
What are the treatments for IC/PBS?
Scientists have not yet found a cure for IC/PBS, nor can they predict who will respond best to which treatment. Symptoms
may disappear with a change in diet or treatments or without explanation. Even when symptoms disappear, they may return after days, weeks, months, or years. Scientists do not know why.
Because the causes of IC/PBS are unknown, current treatments are aimed at relieving symptoms. Many people are helped for variable periods by one or a combination of treatments. As researchers learn more about IC/PBS, the list of potential
treatments will change, so patients should discuss their options with a doctor.
Bladder Distention
Many people with IC/PBS have noted an improvement in symptoms after a bladder distention has been done to diagnose the condition. In many cases, the procedure is used as both a diagnostic test and initial therapy.
Researchers are not sure why distention helps, but some believe it may increase capacity and interfere with pain signals transmitted by nerves in the bladder. Symptoms may temporarily worsen 4 to 48 hours after distention, but should return to predistention levels or improve within 2 to 4 weeks.
Bladder Instillation
During a bladder instillation, also called a bladder wash or bath, the bladder is filled with a solution that is held for varying periods of time, averaging 10 to 15 minutes, before being emptied.
The only drug approved by the U.S. Food and Drug Administration (FDA) for bladder instillation is dimethyl sulfoxide (DMSO, brand name Rimso-50). DMSO treatment involves guiding a narrow tube called a catheter up the urethra into the bladder. A measured amount of DMSO is passed through the catheter into the bladder, where it is retained for about 15 minutes before being expelled. Treatments
are given every week or two for 6 to 8 weeks and repeated as needed. Most people who respond to DMSO notice improvement 3 or 4 weeks after the first 6- to 8-week cycle of treatments. Highly motivated patients who are willing to catheterize themselves may, after consultation with their doctor, be able to have DMSO treatments at home. Self-administration is less expensive and more convenient than going to the doctor’s office.
Doctors think DMSO works in several ways. Because it passes into the bladder wall, it may reach tissue more effectively to reduce inflammation and block pain. It may also prevent muscle contractions that cause pain, frequency, and urgency.
A bothersome but relatively insignificant side effect of DMSO treatments is a garlic-like taste and odor on the breath and skin that may last up to 7 hours after treatment. Long-term treatment has caused cataracts in animal studies, but this side effect has not appeared in humans. Blood tests, including a complete blood count and kidney and liver function tests, should be done about every 6 months.
Oral Drugs
Pentosan Polysulfate Sodium (Elmiron)
This first oral drug developed for IC was approved by the FDA in 1996. In clinical trials, the drug improved symptoms in 30 percent of patients treated. Doctors do not know exactly how the drug works, but one theory is that it may repair defects that might have developed in the lining of the bladder.
The FDA-recommended oral dosage of Elmiron is 100 mg, three times a day. Patients may not feel relief from IC pain for the first to 4 months. A decrease in urinary frequency may take up to 6 months. Patients are urged to continue with therapy for at least 6 months to give the drug an adequate chance to relieve symptoms.
Elmiron’s side effects are limited primarily to minor gastrointestinal discomfort. A small minority of patients experienced some hair loss, but hair grew back when they stopped taking the drug. Researchers have found no negative interactions between Elmiron and other medications.
Elmiron may affect liver function, which should therefore be monitored by the doctor.
Because Elmiron has not been tested in pregnant women, the manufacturer recommends
it not be used during pregnancy, except in the most severe cases.
Other Oral Medications
Aspirin and ibuprofen may be a first line of defense against mild discomfort. Doctors may recommend other drugs to relieve pain.
Some people have experienced improvement in their urinary symptoms by taking tricyclic antidepressants or antihistamines. A tricyclic antidepressant called amitriptyline (Elavil) may help reduce pain, increase bladder capacity, and decrease frequency and nocturia. Some people may not be able to take it because it makes them too tired during the day. In people with severe pain, narcotic analgesics such as acetaminophen (Tylenol) with codeine or longer-acting narcotics may be necessary.
All drugs—even those sold over the counter—have side effects. A person should always consult a doctor before using any drug for an extended amount of time.
Electrical Nerve Stimulation
Mild electrical pulses can be used to stimulate the nerves to the bladder容ither through the skin or with an implanted device. The method of delivering impulses through the skin is called transcutaneous electrical nerve stimulation (TENS). With TENS, mild electric pulses enter the body for minutes to hours, two or more times a day either through wires placed on the lower back or just above the pubic area傭etween the navel and the pubic hair熔r through special devices inserted into the vagina in women or into the rectum in men. Although scientists do not know exactly how TENS relieves pelvic pain, it has been suggested that the electrical pulses may increase blood flow to the bladder, strengthen pelvic muscles that help control the bladder, or trigger the release of substances that block pain.
TENS is relatively inexpensive and allows people with IC/PBS to take an active part in treatment. Within some guidelines, the patient decides when, how long, and at what intensity TENS will be used. It has been most helpful in relieving pain and decreasing
frequency in people with Hunner’s ulcers. Smokers do not respond as well as nonsmokers. If TENS is going to help, improvement is usually apparent in 3 to 4 months.
If TENS is effective in relieving symptoms, a person may consider having a device implanted that delivers regular impulses to the bladder. A wire is placed next to the tailbone and attached to a permanent stimulator under the skin. The FDA has approved this device, marketed as the Inter-Stim system, to treat urge incontinence, urgency-frequency syndrome, and urinary retention in people for whom other treatments have not worked.
Diet
No scientific evidence links diet to IC/PBS, but many doctors and patients find that alcohol, tomatoes, spices, chocolate, caffeinated and citrus beverages, and high-acid foods may contribute to bladder irritation and inflammation. Some people also note that their symptoms worsen after eating or drinking products containing artificial sweeteners. Eliminating various items from the diet and reintroducing them one at a time may determine which, if any, affect a person’s symptoms. However, maintaining a varied, well-balanced diet is important.
Smoking
Many people feel smoking makes their symptoms worse. How the by-products of tobacco that are excreted in the urine affect IC/PBS is unknown. Smoking, however, is the major known cause of bladder cancer. One of the best things smokers can do for their bladder and their overall health is to quit.
Exercise
Many patients feel that gentle stretching exercises help relieve IC/PBS symptoms.
Bladder Training
People who have found adequate relief from pain may be able to reduce frequency by using bladder training techniques. Methods vary, but basically patients decide to void容mpty their bladder預t designated
times and use relaxation techniques and distractions to keep to the schedule. Gradually, they try to lengthen the time between scheduled voids. A diary in which to record voiding times is helpful in keeping track of progress.
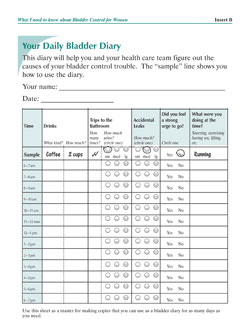
Bladder Diary from What I need to know about Bladder Control for Women at www.kidney.niddk.nih.gov.
Surgery
Surgery should be considered only if all available treatments have failed and the pain is disabling. Many approaches and techniques are used, each of which has advantages and complications that should be discussed with a surgeon. A doctor may recommend consulting another surgeon for a second opinion before taking this step. Most surgeons are reluctant to operate because some people still have symptoms after surgery.
People considering surgery should discuss the potential risks and benefits, side effects, and long- and short-term complications with a surgeon, their family, and people who have already had the procedure. Surgery requires anesthesia, hospitalization, and weeks or months of recovery. As the complexity of the procedure increases, so do the chances for complications and failure.
People should check with their doctor to locate a surgeon experienced in performing specific procedures.
Two procedures—fulguration and resection of ulcers—can be done with instruments inserted through the urethra. Fulguration involves burning Hunner’s ulcers with electricity or a laser. When the area heals, the dead tissue and the ulcer fall off, leaving new, healthy tissue behind. Resection involves cutting around and removing the ulcers. Both treatments are done under anesthesia and use special instruments inserted into the bladder through a cystoscope. Laser surgery in the urinary tract should be reserved for people with Hunner’s ulcers and should be done only by doctors with the special training and expertise needed to perform the procedure.
Another surgical treatment is augmentation, which makes the bladder larger. In most of these procedures, scarred, ulcerated, and inflamed sections of the patient’s bladder are removed, leaving only the base of the bladder and healthy tissue. A piece of the patient’s colon—large intestine—is then removed, reshaped, and attached to what remains of the bladder. After the incisions heal, the patient may void less frequently. The effect on pain varies greatly; IC/PBS can sometimes recur on the segment of colon used to enlarge the bladder.
Even in carefully selected patients—those with small, contracted bladders—pain, frequency, and urgency may remain or return after surgery, and they may have additional problems with infections in the new bladder and difficulty absorbing nutrients from the shortened colon. Some patients become incontinent, while others cannot void at all and must insert a catheter into the urethra to empty the bladder.
Bladder removal, called a cystectomy, is another, infrequently used surgical option. Once the bladder has been removed, different methods can be used to reroute the urine. In most cases, ureters are attached to a piece of colon that opens onto the skin of the abdomen. This procedure is called a urostomy and the opening is called a stoma. Urine empties through the stoma into a bag outside the body. Some urologists are using a second technique that also requires a stoma but allows urine to be stored in a pouch inside the abdomen. At intervals throughout the day, the patient puts a catheter into the stoma and empties the pouch. Patients with either type of urostomy must be very careful to keep the area in and around the stoma clean to prevent infection. Serious potential complications may include kidney infection and small bowel obstruction.
A third method to reroute urine involves making a new bladder from a piece of the patient’s colon and attaching it to the urethra. After healing, the patient may be able to empty the newly formed bladder by voiding at scheduled times or by inserting a catheter into the urethra. Only a few surgeons have the special training and expertise needed to perform this procedure.
Even after total bladder removal, some patients still experience variable IC/PBS symptoms in the form of phantom pain. Therefore, the decision to undergo a cystectomy should be made only after testing all alternative methods and seriously considering the potential outcome.
[Top]
Are there any special concerns regarding IC/PBS?
Cancer
No evidence exists that IC/PBS increases the risk of bladder cancer.
Pregnancy
Researchers have little information about pregnancy and IC/PBS but believe that the disorder does not affect fertility or the health of the fetus. Some women find that their IC/PBS goes into remission during pregnancy, while others experience a worsening of their symptoms.
Coping
The emotional support of family, friends, and other people with IC/PBS is very important in helping patients cope. Studies have found that people who learn about the disorder and become involved in their own care do better than people who do not. The Interstitial Cystitis Association (ICA) maintains a list of support groups.
[Top]
Hope through Research
Although answers may seem slow in coming,
researchers are working to solve the painful riddle of IC/PBS. Some scientists receive funds from the Federal Government to help support their research, while others
receive support from their employing institution, drug pharmaceutical or device companies, or patient support associations.
The National Institute of Diabetes and Digestive and Kidney Diseases・(NIDDK’s) investment in scientifically meritorious IC/PBS research across the country has grown considerably since 1987. The Institute
now supports research that looks at various aspects of IC/PBS, such as how the components of urine may injure the bladder
and what role organisms identified by nonstandard methods may have in causing IC/PBS. In addition to funding research, the NIDDK sponsors scientific workshops where investigators share the results of their studies and discuss future areas for investigation.
Clinical Research Network
The Interstitial Cystitis Clinical Research Network (ICCRN) is a product of two NIDDK programs: the Interstitial Cystitis Database (ICDB) Study and the Interstitial Cystitis Clinical Trials Group (ICCTG). Established in 1991, the ICDB was a 5-year prospective cohort study of more than 600 men and women with symptoms of urinary urgency, frequency, and pelvic pain. The study described the longitudinal changes of urinary symptoms, the impact of IC on quality of life, treatment patterns, and the relationship between bladder biopsy findings and patient symptoms. The ICCTG was established in 1996 as a follow-up to the ICDB study. The clinical trials group developed two randomized, controlled clinical trials of promising therapies, one using oral therapies—pentosan polysulfate sodium (Elmiron) and hydroxyzine hydrochloride (Atarax)—and the other administering intravesical treatment using Bacillus Calmette-Guérin (BCG). BCG is a vaccine for tuberculosis that stimulates the immune system and may have an effect on the bladder. The ICCTG also developed and conducted ancillary studies of various biomarkers such as heparin-binding-growth-factor-like-growth-factor (HB-EGF) and anti-proliferative factor (APF).
In 2003, the ICCTG became the ICCRN, which is conducting additional clinical trials, either sequentially or concurrently, over a second 5-year period. Ancillary studies will be developed and conducted in conjunction with the trials. One of these trials is studying the effectiveness of amitriptyline (Elavil) in treating PBS, which includes IC. Amitriptyline has FDA approval for the treatment of depression, but researchers believe the drug may work to block nerve signals that trigger pain in the bladder and may also decrease muscle spasms in the bladder, helping to cut both pain and frequent urination. Participants in the trial will be randomly assigned to take up to 75 milligrams of amitriptyline or a placebo each day for 14 to 26 weeks.
[Top]
Suggested Reading
The materials listed below may be found in medical libraries, in many college and university libraries, through interlibrary loan in most public libraries, and at bookstores. Items are listed for information only; inclusion does not imply endorsement by the National Institutes of Health.
Articles and Book Chapters
Keay SK, Warren JW. Is interstitial cystitis an infectious disease? International Journal of Antimicrobial Agents. 2002;19(6):480–483.
The Interstitial Cystitis Clinical Trials Group. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. Journal of Urology. 2003;170(3):810–815.
The Interstitial Cystitis Clinical Trials Group. A randomized controlled trial of intravesical Bacillus Calmette-Guérin for treatment of refractory interstitial cystitis. Journal of Urology. 2005;173(4):1186–191.
Books and Booklets
Moldwin RM. Interstitial cystitis survival guide: your guide to the latest treatment options and coping strategies. Oakland, CA: New Harbinger Publications, Inc.; 2000. (Available by calling 1–800–HELP–ICA.)
Sandler GG, Sandler A. Patient to patient: managing interstitial cystitis and overlapping conditions. New Orleans, LA: Bon Ange LLC; 2000.
Sant G, ed. Interstitial cystitis. Philadelphia: Lippincott-Raven; 1997.
The U.S. Government does not endorse or favor any specific commercial product or company. Trade, proprietary, or company names appearing in this document are used only because they are considered necessary in the context of the information provided. If a product is not mentioned, the omission does not mean or imply that the product is unsatisfactory.
[Top]
For More Information
American Urological Association Foundation
1000 Corporate Boulevard
Linthicum, MD 21090
Phone: 1–866–RING–AUA (746–4282) or 410–689–3700
Email: patienteducation@auafoundation.org
Internet: www.auafoundation.org
www.UrologyHealth.org
American Chronic Pain Association
P.O. Box 850
Rocklin, CA 95677
Phone: 1–800–533–3231
Email: ACPA@pacbell.net
Internet: www.theacpa.org
American Pain Society
4700 West Lake Avenue
Glenview, IL 60025
Phone: 847–375–4715
Email: info@ampainsoc.org
Internet: www.ampainsoc.org
American Urogynecologic Society
2025 M Street NW., Suite 800
Washington, DC 20036
Phone: 202–367–1167
Fax: 202–367–2167
Email: augs@dc.sba.com
Internet: www.augs.org
International Association for the Study of Pain
111 Queen Anne Avenue North, Suite 501
Seattle, WA 98109–4955
Phone: 206–283–0311
Email: iaspdesk@iasp-pain.org
Internet: www.iasp-pain.org
Interstitial Cystitis Association
110 North Washington Street, Suite 340
Rockville, MD 20850
Phone: 1–800–HELP–ICA (435–7422) or 301–610–5300
Fax: 301–610–5308
Email: ICAmail@ichelp.org
Internet: www.ichelp.org
National Kidney Foundation, Inc.
30 East 33rd Street
New York, NY 10016
Phone: 1–800–622–9010 or 212–889–2210
Internet: www.kidney.org
National Organization of Social Security
Claimants’ Representatives
560 Sylvan Avenue
Englewood Cliffs, NJ 0763
Phone: 1–800–431–2804
Email: NOSSCR@att.net
Internet: www.nosscr.org
Social Security Administration
Office of Public Inquiries Windsor Park Building
6401 Security Boulevard
Baltimore, MD 21235・401
Phone: 1–800–772–1213
Internet: www.ssa.gov
Local offices can be found in the telephone book under U.S. Government, Department of Health and Human Services.
United Ostomy Association
19772 MacArthur Boulevard, Suite 200
Irvine, CA 92612
Phone: 1–800–826–0826 or 949–660–8624
Fax: 949–660–9262
Email: info@uoa.org
Internet: www.uoa.org
The National Kidney and Urologic Diseases Information Clearinghouse collects resource information about kidney and urologic diseases for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Reference Collection. This database provides titles, abstracts, and availability information for health information and health education resources. The NIDDK Reference Collection is a service of the National Institutes of Health.
You may view the results of the automatic search on Interstitial Cystitis.
If you wish to perform your own search of the database, you may access and search the NIDDK Reference Collection database online.
This publication may contain information about medications used to treat a health condition. When this publication was prepared, the NIDDK included the most current information available. Occasionally, new information about medication is released. For updates or for questions about any medications, please contact the U.S. Food and Drug Administration at 1–888–INFO–FDA (463–6332), a toll-free call, or visit their website at www.fda.gov. Consult your doctor for more information.
[Top]
National Kidney and Urologic Diseases Information Clearinghouse
3 Information Way
Bethesda, MD 20892–3580
Phone: 1–800–891–5390
TTY: 1–866–569–1162
Fax: 703–738–4929
Email: nkudic@info.niddk.nih.gov
Internet: www.kidney.niddk.nih.gov
The National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC) is a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIDDK is part of the National Institutes of Health of the U.S. Department of Health and Human Services. Established in 1987, the Clearinghouse provides information about diseases of the kidneys and urologic system to people with kidney and urologic disorders and to their families, health care professionals, and the public. The NKUDIC answers inquiries, develops and distributes publications, and works closely with professional and patient organizations and Government agencies to coordinate resources about kidney and urologic diseases.
Publications produced by the Clearinghouse are carefully reviewed by both NIDDK scientists and outside experts.
This publication is not copyrighted. The Clearinghouse encourages users of this publication to duplicate and distribute as many copies as desired.
NIH Publication No. 08–3220
April 2008
[Top]
|






