
Cancer Imaging Looks Beyond Jellyfish Genes
Jellyfish are drifters who mostly go where the currents take them. But the animals deserve some credit for sparking a revolution in how scientists track the movements and interactions of proteins in many kinds of cells, including cancer cells.
 A green fluorescent protein was isolated from the jellyfish Aequorea victoria, above, in 1962. Copyright Sierra Blakely
A green fluorescent protein was isolated from the jellyfish Aequorea victoria, above, in 1962. Copyright Sierra Blakely
Recognition came last year in the form of a Nobel Prize. Three scientists were honored for discovering the green fluorescent protein (GFP) in the jellyfish Aequorea victoria and turning it into a powerful research tool. In mice and other animals, the glow of GFP has illuminated aspects of biology that were long hidden from view, including the spread of cancer, the Nobel committee noted.
The magic of GFP is that the protein can glow outside of jellyfish. In a common experiment, GFP is genetically linked to another protein so that the activation of the linked protein turns on GFP as well, producing a telltale glow under the right light.
Since the first GFP was discovered in 1962, additional types and colors have been found in nature or developed in the lab. Recent studies have expanded the limits of what can be observed, and they have shown how this technology might be translated into a powerful imaging tool in the clinic.
Watching Cancer Spread
Scientists have used GFP to observe the spread of cancer cells in mice for up to several days, but a new method extends this observation period to weeks. The technique involves shining a special light on tumor cells that were engineered to produce a GFP-like protein derived from coral. When hit by the light, the cells turn from green to red, making it possible to track their movement.
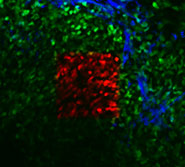 A mammary tumor in a living mouse as seen through a mammary imaging window. Tumor cells are green and optically-marked tumor cells are red. The extracellular matrix is blue. (Image courtesy of Dr. Jacco van Rheenen)
A mammary tumor in a living mouse as seen through a mammary imaging window. Tumor cells are green and optically-marked tumor cells are red. The extracellular matrix is blue. (Image courtesy of Dr. Jacco van Rheenen)
As a demonstration, researchers in the Gruss Lipper Biophotonics Center (GLBC) at Albert Einstein College of Medicine tracked two populations of cells from the same breast tumor over several weeks. Mice in the study had “imaging windows” inserted into their mammary glands so that the researchers could easily find the cells at each inspection.
“Now that we can optically mark tumor cells by turning them from green to red, we can start to map the fates of these cells in the body,” said Dr. John Condeelis of GLBC, who co-led the study with his colleague Dr. Jeff Segall. Their findings appeared in the December 2008 Nature Methods.
The study showed that tumor cells near blood vessels were more likely to spread to the lungs than other tumor cells, suggesting the importance of the local environment in the spread of cancer. “Even within the same tumor there were regions where you get different types of metastases,” said senior author Dr. Jacco van Rheenen, who is currently at the Hubrecht Institute in the Netherlands.
Imaging Probes
While the GFP toolbox has grown, the technology has the limitation that a protein must be encoded in the genome before it is produced. For use in patients with cancer, fluorescent compounds would likely have to be injected or given orally. A new study by researchers at NCI and in Japan suggests how this might work.
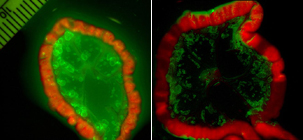 An activatable probe (right) shows a metastatic ovarian tumor with minimal background signals. The control "always on" probe (left) lacks clear delineation of cancer foci due to high background signals. (Image courtesy of Dr. Hisataka Kobayashi)
An activatable probe (right) shows a metastatic ovarian tumor with minimal background signals. The control "always on" probe (left) lacks clear delineation of cancer foci due to high background signals. (Image courtesy of Dr. Hisataka Kobayashi)
The team developed a fluorescent compound that can be attached to a tumor-specific antibody and only lights up when the antibody is taken inside living cancer cells. When the compound was tested in mice using the breast cancer drug trastuzumab (Herceptin), the “probe” almost always hit its target.
“All we see are the healthy cancer cells,” said Dr. Hisataka Kobayashi of the Molecular Imaging Program at NCI's Center for Cancer Research (CCR). He developed a pH-sensitive antibody with Dr. Yasuteru Urano, a chemist at the University of Tokyo. Their study appears this month in Nature Medicine.
Most imaging probes produce a signal even after a cell is damaged or dies, and they also bind to normal cells. This could be misleading to physicians who want to identify viable cancer cells. The new probe was designed to be activated exclusively in cells that pull it into a part of the cell called the lysosome. Because the lysosome maintains an acidic environment in healthy cells, a pH-sensitive probe such as this is largely active only in living cancer cells.
Into the Clinic
If developed further, the technology could possibly be used to diagnose cancer and monitor the effects of therapy almost in real time, said Dr. Kobayashi. Another potential application would be to guide surgeons as they try to remove tumor tissue from patients with cancers, he added.
As a step toward the clinic, NCI is collaborating on a clinical trial to test different imaging tools, including activatable probes, as a way to improve the treatment and detection of ovarian cancer.
“With this disease, we know that better surgeries to remove the cancer lead to better outcomes,” said coauthor Dr. Peter Choyke, who directs the Molecular Imaging Program in CCR. “These activatable probes may improve the ability to detect the earliest signs of the disease and to improve outcomes.”
—Edward R. Winstead