
Lung Cancer and Erlotinib: Which Patients Benefit?
One of the most promising recent developments in cancer medicine has been the dramatic responses of some patients with lung cancer to the drugs gefitinib (Iressa) and erlotinib (Tarceva). Although only about 10 percent of patients with lung cancer respond in this way, a larger group experiences prolonged survival and an improvement in symptoms. Unfortunately, the best way to identify candidates for these drugs, called EGFR tyrosine kinase inhibitors (TKIs), is not yet clear.
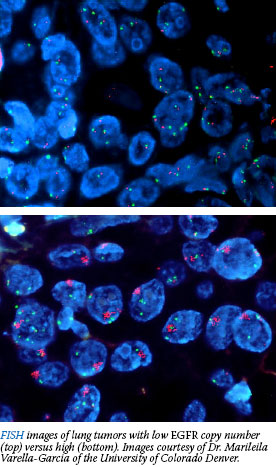 NCI has launched a clinical trial called MARVEL (Marker Validation of Erlotinib in Lung Cancer) that could offer guidance. The study aims to validate a candidate biomarker for response to erlotinib: extra copies of the epidermal growth factor receptor (EGFR) gene in lung tumors. Several recent studies have suggested that patients who harbor this gene "amplification" may benefit from erlotinib. The trial will also assess other candidate prognostic markers such as EGFR gene mutations. NCI has launched a clinical trial called MARVEL (Marker Validation of Erlotinib in Lung Cancer) that could offer guidance. The study aims to validate a candidate biomarker for response to erlotinib: extra copies of the epidermal growth factor receptor (EGFR) gene in lung tumors. Several recent studies have suggested that patients who harbor this gene "amplification" may benefit from erlotinib. The trial will also assess other candidate prognostic markers such as EGFR gene mutations.
"This study represents a unique opportunity for the prospective validation of multiple markers," said co-principal investigator Dr. Fred R. Hirsch of the University of Colorado Health Sciences Center. He and the group at the University of Colorado Cancer Center pioneered the use of fluorescence in situ hybridization (FISH) as a means of detecting EGFR amplifications in lung tumors, and the technology will be evaluated in MARVEL along with the biomarker itself.
Breast cancer tumors are routinely analyzed using FISH to identify women who are candidates for trastuzumab (Herceptin), and the technology could readily be adapted for clinical use in lung cancer.
In the randomized trial, approximately 1,200 patients with advanced non-small-cell lung cancer (NSCLC) who were treated previously will receive erlotinib or pemetrexed, a standard second-line chemotherapy drug for lung cancer. The investigators hypothesize that erlotinib will be superior in patients who are EGFR-positive as detected by FISH, while the chemotherapy will be superior in those who test EGFR-negative.
"This study has two main purposes - to define which population will benefit the most from receiving EGFR-targeted therapies, and to validate some of the tests used to measure the EGFR target," said Dr. Claudio Dansky Ullmann of NCI's Division of Cancer Treatment and Diagnosis, who, along with Dr. Janet Dancey, formerly of NCI and now at the Ontario Institute for Cancer Research, helped develop the trial.
Multiple cancers involve the activation of the EGFR gene, either through mutation or increases in the number of gene copies of EGFR. Though EGFR gene mutations are associated with strong responses to erlotinib, sometimes lasting several years, the mutations are rare in Western populations. By comparison, approximately 50 percent of NSCLCs may harbor increased EGFR gene copy number. This suggests that the change, if validated, could be broadly useful as a biomarker of response, noted Dr. Hirsch.
"Our hope is that the trial will lead to the identification of markers that will ultimately allow the selection of a specific treatment that is likely to be the most effective for individual patients," said Dr. Dancey.
Along with molecular markers, clinical characteristics such as being a female, Asian ethnicity, and smoking fewer than 100 cigarettes in a lifetime are associated with response to EGFR TKIs. Responders also tend to have lung adenocarcinomas.
The trial will also evaluate mutations in the KRAS gene as possible biomarkers. In colorectal cancer, KRAS mutations are associated with resistance to cetuximab (Erbitux), a monoclonal antibody that targets the EGFR protein, but there is insufficient evidence to draw conclusions about these mutations in lung cancer.
"We expect that this study will teach us a lot about how the different EGFR biomarkers correlate to each other," said Dr. Hirsch, "and also about their associations with clinical outcomes."
The multicenter study is NCI's first to determine prospectively whether biomarkers can help guide treatment for lung cancer. Many partners have been involved in the planning and design from the outset, including NCI cooperative groups, the Food and Drug Administration (FDA), and the Centers for Medicare and Medicaid Services.
"The MARVEL trial is unique and an important first because it is an outgrowth of specific NCI initiatives designed to advance lung cancer therapies and received broad input from the FDA, NCI cooperative groups, the biomarker industry, and the pharmaceutical industry," said Dr. Alex A. Adjei, of the Roswell Park Cancer Institute and chair of the study, in a statement.
—Edward R. Winstead
|