|
|
|
|||||||||||

|
|||||||||||||||||||||||||||||||||||||||||||||
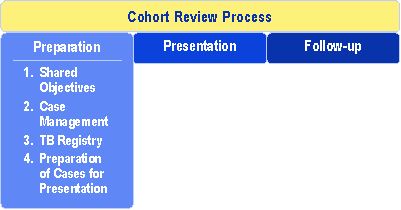
Understanding the TB Cohort Review Process: Instruction Guide 2006Essential Element 1: Preparation for a Cohort ReviewThis chapter provides information for preparing for a cohort review session. Each of the four areas of cohort review preparation is explained in detail, and the necessary tools are provided. For some areas, case studies or exercises are suggested to help individual readers or groups learn necessary skills. |
| Read |
|
|---|---|
| Insert |
|
| Insert |
|
- Are there any new objectives your program area would like to set? If so, what are they?
For a cohort review, the objectives become the standards to which the outcomes of case management and contact investigation efforts are compared. The objectives also dictate which data elements need to be collected in the TB registry and presented at the cohort review session. For example, in order to determine what percentage of contacts of smear-positive patients were evaluated, data must be collected on smear results, number of contacts identified, and number of contacts evaluated.
After the cohort review, if some new aspect of the program needs to be strengthened, objectives can be revised or new objectives can be added for the next cohort review. For example, one TB program believed that the timeliness of conducting the first interview with a newly diagnosed TB patient was an area that needed improvement. Therefore they set an objective that was SMART, an objective that is specific, measurable, attainable, realistic, and time-framed. The objective was that “At least 90% of persons identified with TB disease will be interviewed by health department staff within 3 business days of case notification.” Starting at the next cohort review, the date the case was reported and the date of the initial interview were part of the standard case presentation format. By adding these data elements, the program was able to track this timeliness variable and measure improvement over time.
2. Comprehensive Case Management
Comprehensive case management is essential in TB control and elimination efforts. Cohort review brings together data from many of the components of case management and provides a qualitative assessment of the effectiveness of case management activities.
Case management is a system in which a specific health department employee, typically a case manager, is assigned primary responsibility for managing the patient’s case. Systematic, regular review of patient progress is conducted, and plans are made to address any barriers to adherence. All reported cases should be assigned to a case manager, whether they are seen at a health department clinic or in the private sector. The case manager is responsible for ensuring that patients adhere to treatment, comply with medical visits, and complete treatment. In addition, case managers are also responsible for making sure that contacts are identified and evaluated, and that they complete treatment for LTBI, if appropriate.
In general, case managers are expected to
- Follow all policies and protocols for case management to ensure that patients adhere to treatment, comply with medical visits, and complete treatment.
- Follow all policies and protocols for contact investigation to ensure that contacts are identified and evaluated, and that they complete treatment for LTBI if appropriate.
- Communicate periodically with clinic and outreach workers to ensure all aspects of patient care are being addressed and troubleshoot any problems that arise.
- Participate in case review meetings with their supervisor and the TB control team.
- Prepare information on each case, present the information at the cohort review session, and follow up on suggestions made at the cohort review session.
Exercise 3: Reviewing Case Management Protocols
| Discuss the following questions with your TB control team.
|
For more information on case management protocols and training, see the Resources section at the end of this document.
Improvement. Therefore they set an objective that was SMART, an objective that is specific, measurable, attainable, realistic, and time-framed. The objective was that “At least 90% of persons identified with TB disease will be interviewed by health department staff within 3 business days of case notification.” Starting at the next cohort review, the date the case was reported and the date of the initial interview were part of the standard case presentation format. By adding these data elements, the program was able to track this timeliness variable and measure improvement over time.
3. Reliable TB Registry
A reliable TB registry is an essential tool of the cohort review process. Typically, programs use a registry database to collect TB patient and contact investigation information. A locally developed database provides the universe of patients from which the cohort is drawn. The date on which the case is counted determines the cohort in which the case will be reviewed.
A reliable TB registry will include
|
The data analyst will use the registry to generate the list of TB cases to be reviewed in a given cohort, being certain to include the data elements needed to evaluate program objectives. He or she will distribute the lists to case managers and supervisors so they can be prepared to present these cases.
The following lists are prepared and distributed ahead of time:
- Preliminary cohort list. Distributed 5–6 months before a cohort review. This list provides diagnostic and preliminary treatment and contact information. The supervisor and case manager use this list to track the cohort of patients from a quarter and to begin preparation of case presentations for a cohort review.
- Final cohort list. Distributed 1–2 months before a cohort review. This list provides updated treatment information from the registry and the results of the contact investigation. The supervisor and case managers use this list to hold practice review sessions and complete final preparation of the case presentations.
Typically, a “line listing” is all that is needed—one line for each case. The information may be more complete for the final cohort list because more time has elapsed.
See:
- Table 10: Sample of Preliminary Cohort List and
- Table 11: Sample Final Cohort List: on the following two pages.
Last Reviewed: 05/18/2008
Content Source: Division of Tuberculosis Elimination
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention
If you would like to order any of the DTBE publications please visit the online order form.
You will need Adobe Acrobat™ Reader v5.0 or higher to read pages that are in PDF format. Download the Adobe Acrobat™ Reader.
If you have difficulty accessing any material on the DTBE Web site because of a disability, please contact us in writing or via telephone and we will work with you to make the information available.
Division of Tuberculosis Elimination
Attn: Content Manager, DTBE Web site
Centers for Disease Control and Prevention
1600 Clifton Rd., NE Mailstop E-10
Atlanta, GA 30333
CDC-INFO at (1-800) 232-4636
TTY: 1 (888) 232-6348
E-mail: cdcinfo@cdc.gov
![]() Home | Site Map
| Contact Us
Home | Site Map
| Contact Us
Accessibility
| Privacy Policy Notice |
FOIA
| USA.gov
CDC Home |
Search |
Health Topics A-Z
Centers for Disease Control & Prevention
National Center for HIV/AIDS,
Viral Hepatitis, STD, and TB Prevention
Division of Tuberculosis Elimination
Please send comments/suggestions/requests to: CDCINFO@cdc.gov