


   |
| FDA Home Page | CDRH Home Page | Search | A-Z Index |  |
|
 |
 |

|
U.S. Department
of Health and Human Services |
Les Weinstein was appointed as the first CDRH Ombudsman in April 2000. As an external Ombudsman, he looks into complaints from outside the agency and facilitates the resolution of disputes between CDRH and the medical device industry it regulates. While providing this service, he maintains his impartiality and neutrality. This Annual Report focuses on complaints and disputes: the number of contacts the Ombudsman received, their source, the CDRH offices involved, the subjects, reasons and disposition. You may also wish to see his web site at: http://www.fda.gov/cdrh/resolvingdisputes/ombudsman.html.
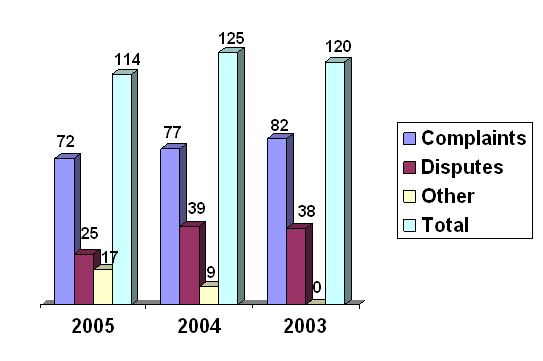
Most contacts the Ombudsman receives are in the form of a complaint or dispute. A complaint is typically an expression of dissatisfaction, perhaps about timeliness, lack of communication, or an unhelpful employee. A dispute usually involves a disagreement with, a challenge to, or an appeal of a decision or action the Center has taken or is about to take.
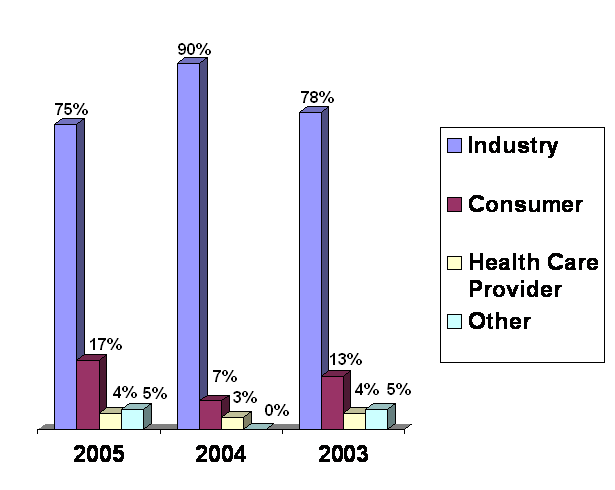
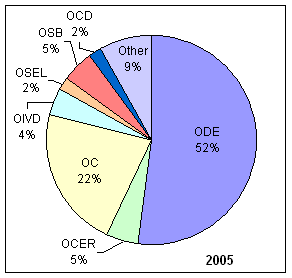
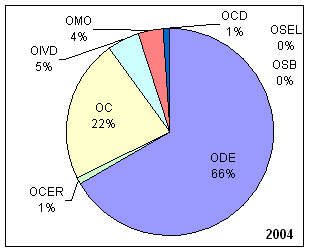
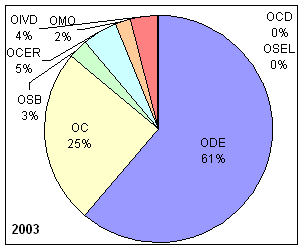
Key:
| OCD | Office of Center Director | OC | Office of Compliance |
| OCER | Office of Communication, Education, and Radiation Programs | ODE | Office of Device Evaluation |
| OIVD | Office of In Vitro Diagnostic Device Evaluation and Safety | OMO | Office of Management Operations |
| OSEL | Office of Science and Engineering Laboratories | OSB | Office of Surveillance and Biometircs |
|
2005 | 2004 | 2003 |
|---|---|---|---|
| 510(k) | 16% | 28% | 25% |
| Adverse Event/MDR/Device Malfunction | 9% | N/A | N/A |
| Imports | 8% | 7% | 6% |
| PMA | 7% | 6% | 9% |
| IDE | 6% | 6% | 4% |
| 513g | 5% | 6% | 4% |
| Disclosure | 4% | N/A | N/A |
| Trade Complaint | 4% | 7% | 6% |
| Data Integrity | 3% | N/A | N/A |
| Exports | 3% | 3% | 1% |
| SUD/Re-use/Reprocessing | 3% | N/A | N/A |
| Whistle Blower | 3% | N/A | N/A |
| Combination Product | 3% | 2% | 2% |
| Laser | 3% | N/A | N/A |
| Mammography | 2% | N/A | N/A |
| Pre-IDE | 2% | N/A | N/A |
| Promotion | 2% | N/A | N/A |
| Software | 2% | N/A | N/A |
| 2005 | 2004 | 2003 |
|---|---|---|
| 1. Miscommunication or lack of communication | 1 | 1 |
| 2. Data, testing requirements to support a submission; “least burdensome” | 2 | 3 |
| 3. Lack of timeliness (of approval/clearance; setting up meetings; returning phone calls; etc.) | 4 | 2 |
| 4. Safety concern/issue | 8 | 7 |
| 5.Various policies and procedures | 3 | 4 |
| 6. Level playing field (claim of unequal treatment) | 5 | 5 |
| 7. Difficult or unhelpful employee | 6 | 6 |
| 8. Freedom of Information Act (FOIA) | N/A | N/A |
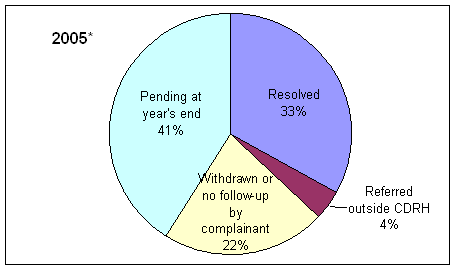
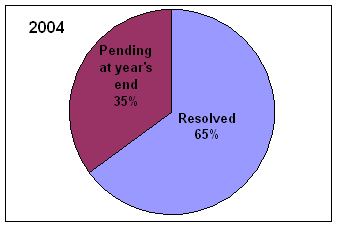
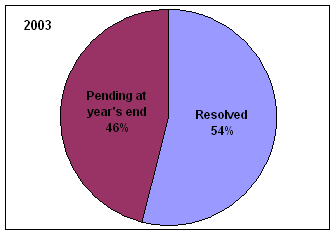
* Includes complaints/disputes received in 2005 plus those pending from previous years that were carried over to 2005.
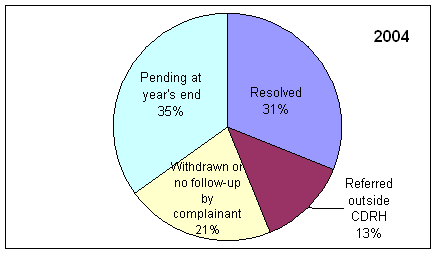
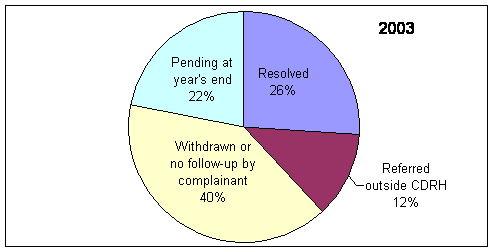
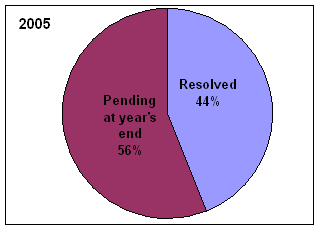
* Includes complaints/disputes received in 2005 plus those pending from previous years that were carried over to 2005.
II. MEDICAL DEVICES DISPUTE RESOLUTION PANEL
On July 2, 2001 the Guidance for industry and FDA entitled “Resolving Scientific Disputes Concerning the Regulation of Medical Devices: a Guide to Use of the Medical Devices Dispute Resolution Panel” was issued in Final. This Guidance is available at http://www.fda.gov/cdrh /resolvingdisputes/1121.pdf.
In 2005 and 2004 the Ombudsman did not receive any requests for review of a scientific dispute by the Medical Devices Dispute Resolution Panel. In 2003 the Ombudsman received one request, which he granted, but the scheduled meeting of the Panel was subsequently postponed indefinitely.
Updated June 7, 2006
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH