CBER Presentation
Prospects for DNA-Based Tests to Detect Malaria Infections
FDA Workshop on Testing for Malarial Infections in Blood Donors
July 12, 2006
Sanjai Kumar, Ph.D.
Center for Biologics Research and Review
Food and Drug Administration
DNA-Based Tests for the Detection of Malaria Parasites
- DNA-based tests are the mainstay of blood donor screening method to detect infections with HIV, HCV, and West Nile Virus
- Why such a blood screening test is not available for malaria?
- A large number of publications in malaria-
- Routine diagnosis
- Monitor the efficacy of drugs
- Genotyping
- Vaccine efficacy
Challenges and Considerations
- Malaria parasites are highly infectious - 10 blood form P. vivax parasites are sufficient to cause a virulent infection
- Window period in non-immune travelers
- Minimum parasite burden in asymptomatic donors is not known and, therefore the absolute required assay sensitivity is difficult to predict
- The number of parasites that survive between the time of donation to transfusion is not known
- How to detect to a few parasites potentially present in a unit of blood?
DNA-Based Tests for the Detection of Malaria Parasites
- PCR-amplification (primary and nested-PCR)
- TaqMan assay, Real-time PCR
- Microarray test
- 18 S rRNA gene is the primary target
- First malaria rRNA gene isolated by Tom McCutchan in 1983
- RNA based diagnosis of P. falciparum. McCutchan group. Lancet 1989
- 7-8 copies of the gene per genome
- Contains both conserved and semi-conserved regions allowing Plasmodium species identification
Detection Limit of the TaqMan Assay for P. falciparum Parasites Spiked in Normal Blood
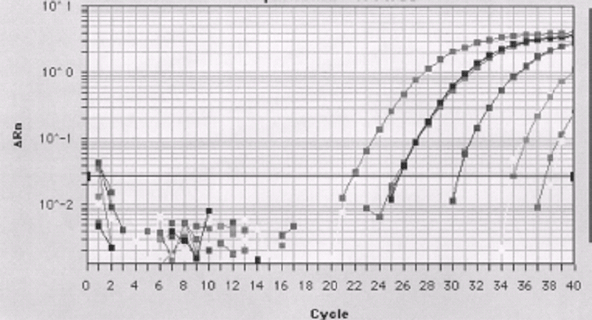
Detection limit - 1 parasite/mL of blood
Microarray Test
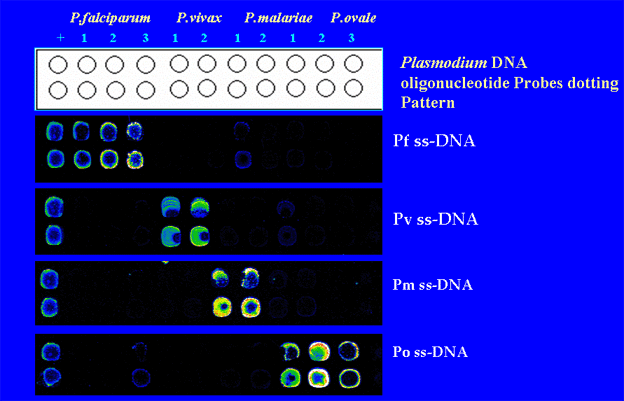
DNA based tests for ring form Plasmodium falciparum Detection using 18s rRNA by Nested PCR

Whole Blood Boiling Method
- Detects 0.25 parasite/µl of blood
- Potentially could leave up to 112, 500 parasites undetected in a unit of blood
DNA Isolation QIAamp Blood Kit
- Detects up to 2.0 parasites/ml of blood
- Potentially could leave up to 900 parasites undetected in a unit of blood
DNA-Based Tests for the Detection of Malaria Parasites*
- Non-radioactive PCR hybridization (whole blood). Drug efficacy trial: 20 parasites/ml. Ciceron et al. JCM 1999
- Quantitative real-time PCR (genomic DNA isolation). Vaccine efficacy trial: 20 parasites/ml. Andrews et al. AJTMH 2005
- Nest-PCR (genomic DNA isolation). Parasite spiking study: 2 parasites/ml, S Kumar, FDA (unpublished)
*Data based on a few published reports
Determining the Time of Sporozoite Exposure to Appearance of Blood Form Parasites (window period) by a PCR Test
- Vaccine efficacy study. Following sporozoite challenge, real-time PCR can detect malaria parasite in the blood up to 5 days before microscopy. Andrews et al. AJTMH 2005
- It is reasonable to predict that real-time PCR could be used to detect malaria parasites in the blood of the majority of non-immune travelers two-weeks after departing an endemic area
Prospects for DNA-Based tests for Blood Donor Screening for Malaria Infections
- Highly infectious nature of malaria parasites causes a potential risk from a few parasites that could be present in a unit of blood
- Highest sensitivity achieved: 2 to 20 parasites/ml
- Minimum number of infectious parasites present in a unit of blood: Not known (biggest road block)
- Possible solutions:
- A technology for parasite concentration
- An accurate knowledge of the minimum parasite burden in infected donors - that would allow to determine the required assay sensitivity
Acknowledgements
- CBER
Hong Zheng
Babita Mahajan
Vladimir Chizhikov
- WRAIR
David Haynes


