Pandemic Planning Update VI
A scant 33 months ago, I sent my first message about a race that HHS had just begun. As I said then, it was a race against a fast-moving virulent virus with the potential to cause an influenza pandemic. Since then, we have mobilized experts and resources across the country and around the world. I now send you this final message, as I look back at the unprecedented progress we have made in energizing a national pandemic influenza preparedness movement in those 33 months.
“The history of pandemics is not so much the history of health as it is the history of humanity. When pandemics strike, they cause massive sickness and terrible loss of life. They even reshape nations.”
– HHS Secretary Mike Leavitt
Today, many people mistakenly think influenza pandemics are a thing of the past, but influenza has struck hard in the era of modern medicine – much harder than most people realize. And it will strike again. Pandemics are hard things to talk about. When one discusses them in advance, it sounds alarmist. After a pandemic starts, no matter how much preparation has been done, it will be inadequate.
In November 2005, President Bush mobilized the nation to prepare for an influenza pandemic. He called for the deployment of a $7.1 billion national pandemic plan. Congress responded quickly. As Secretary of HHS, I was given responsibility to implement the plan.
 Ultimately, the key to preparing for a pandemic is to develop, stockpile, and prepare to distribute vaccines and antivirals – vaccines to prevent people from becoming infected by a virus, and antivirals to treat them if they are infected. But, how to achieve this? Developing and stockpiling vaccines is not a job for any one government. It is not even a job for any one nation.
Ultimately, the key to preparing for a pandemic is to develop, stockpile, and prepare to distribute vaccines and antivirals – vaccines to prevent people from becoming infected by a virus, and antivirals to treat them if they are infected. But, how to achieve this? Developing and stockpiling vaccines is not a job for any one government. It is not even a job for any one nation.
Rather, it requires cooperation among nations, cooperation among different government entities within nations, and cooperation between governments and the private sector. Pandemic preparedness requires that all of these different elements work together. The better they do so, the better prepared we will be as a nation.
Local preparedness is the foundation of pandemic readiness. In addition to State governors, leadership must come from mayors, county commissioners, school principals, faith leaders, college presidents, corporate planners, and the entire healthcare infrastructure.
One of our highest priorities is to enhance and expand U.S.-based vaccine production capacity to the point that it can generate enough pandemic influenza vaccine for every American within six months of the time that the actual pandemic virus is identified, wherever in the world it is identified.
 Today with over $1 billion of HHS funding, six companies are in various stages of implementing commercial-scale production of cell-based methods and/or expanding their capacity for conventional manufacturing using chicken eggs. We will reach our goal of having the needed capacity by 2011.
Today with over $1 billion of HHS funding, six companies are in various stages of implementing commercial-scale production of cell-based methods and/or expanding their capacity for conventional manufacturing using chicken eggs. We will reach our goal of having the needed capacity by 2011.
Partly as a result of these efforts, we have now procured 12.2 million treatment courses (each course provides a full treatment for one person) of H5N1 pre-pandemic influenza vaccine. If the virus mutates to where it has pandemic properties, a new vaccine will be developed to match it exactly, but this stockpiled vaccine will provide some protection in the interim.
This stockpile is available to support clinical trials and to protect healthcare workers, first responders, and other critical workers in the early stages of a pandemic. We plan to stockpile additional doses of pre-pandemic vaccine by the end of 2008.
We have reached our goal of stockpiling enough pandemic influenza antivirals to cover 44 million people, which will help slow the spread of an emerging pandemic.
“The Federal government has a role, as do state and local governments, not-for-profit organizations, the business community large and small. We are all in this together. The challenge remains.”
– Dr. William Raub,
Science Advisor to the HHS Secretary
State governments have taken advantage of a chance to purchase up to 31 million treatment-courses of antiviral drugs while receiving a 25 percent federal subsidy and while making use of federal "best price" contracts. Some states have also purchased additional antiviral drugs, using these federal contracts, with unsubsidized funds. To date, the States have purchased 22 million treatment courses of Tamiflu® and Relenza® pandemic stockpiles.
We have awarded a total of $600 million for State and local preparedness. Through these grants, HHS has assisted in building response elements in local communities and has supported expanded interoperability. This means States can help each other on an almost interchangeable basis.
 We have worked with States and local communities to update their pandemic influenza plans and to ensure that they evaluate their plans by conducting exercises. To make sure that States knew the importance of this effort, we conducted a pandemic influenza summit in each of the fifty United States and every one of the U.S. territories. I personally attended about three-quarters of the State summits. The summits brought together government representatives with healthcare professionals, schools, and the private sector, to advance State and community preparedness.
We have worked with States and local communities to update their pandemic influenza plans and to ensure that they evaluate their plans by conducting exercises. To make sure that States knew the importance of this effort, we conducted a pandemic influenza summit in each of the fifty United States and every one of the U.S. territories. I personally attended about three-quarters of the State summits. The summits brought together government representatives with healthcare professionals, schools, and the private sector, to advance State and community preparedness.
In addition to all of this, we purchased more than 150 million masks and respirators. We have obligated $100 million for the purchase of ventilators, syringes, intravenous antibiotics, and other supplies for potential distribution in case of an influenza pandemic. We have developed a highly sensitive rapid diagnostic test for avian and seasonal influenza. This will serve as the gold standard worldwide for confirming the first cases of avian or potentially pandemic influenza.
We have awarded funds to developers of new point-of-care influenza rapid test devices. These will enable doctors and other health-care professionals to diagnose avian influenza rapidly and easily in a clinical office or other outpatient setting. Clinical trials and FDA approval are anticipated by the end of 2009.
We have supported pandemic influenza preparedness activities in approximately 40 countries around the world. Since 2005, HHS has committed over $350 million internationally. This assistance is helping other countries improve their laboratory capacities, develop and strengthen disease-surveillance systems, develop preparedness plans, and train health workers to respond rapidly to outbreaks.
We have deployed teams of experts to help investigate suspected cases of possible human transmission of bird flu in 12 countries in Asia, Africa, and Europe. We have led workshops to provide country-level rapid response training in 112 countries, from all six regions of the World Health Organization (WHO).
In summary, we are much better prepared today than we were in the spring of 2005. But much still needs to be done. As I turn over this responsibility to the next Secretary of HHS, there are four priorities I would suggest they consider.
First, finish the vaccine manufacturing facilities. By the end of 2008, we plan to award approximately $500 million in contracts to companies that will build cell-based vaccine production facilities in the United States.
Second, strongly defend the global influenza virus sample-sharing network. For sixty years, nations have openly and freely shared virus samples. It has been one of the most important global health success stories, and it is being threatened right now by a few nations.
Third, concentrate on countermeasure distribution. We have developed an impressive stockpiling system that will allow the deployment of federal supplies in the early stages of a pandemic emergency. However, getting pills into the palms of people’s hands fast is critical, and not every State has adequate plans to carry that out.
And, finally, my last suggestion is directly related: continually remind States, communities, businesses, and families about their responsibility to be prepared. This is a never-ending task. The media buzz has died down, but the “bird flu” virus has not.
The federal government has an indispensable role to do things that no individual state can do on its own, and to provide added capacity and coordination. In the last three years, there has been tremendous progress in the federal government’s capacity to meet its indispensable role.
However, local and State governments know their communities; they understand their unique needs. The key to success in preparing for a pandemic or any potential disaster is collaboration.
Preparation is a continuum. Each day that we prepare brings us closer to being ready. We are better prepared today than we were yesterday. And we will be better prepared tomorrow than we are today.
I believe we can prepare successfully for a pandemic. But it will require determination. It will require change.
Thank you.
top of page
The story of our country’s pandemic preparations is, at its center, a story about individuals and their sense of commitment to a common goal. It is a story of shared responsibility, of people working together to prepare their families, their communities, workplaces, cities, and states for the eventuality of a pandemic.
At each level of this combined effort, the Department of Health and Human Services (HHS) has taken steps so that those involved had the information, guidance, and support to work together to prepare for a pandemic. Individuals, too, within the Federal government are working together to take the broader actions that can only be done from a central, national vantage point.
After three years of intense effort by these individuals and groups throughout the country, many results of this unique national effort are now emerging. At the same time, the next steps needed to continue this effort and improve upon it are coming into sharper focus.
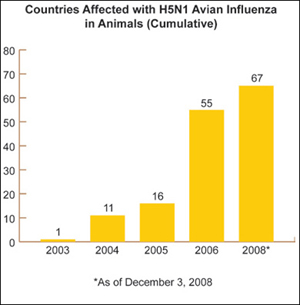
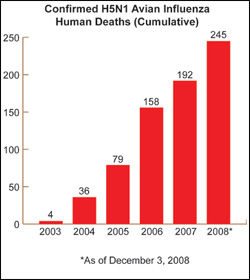
As part of the strategy to diminish the global risk of pandemic influenza, HHS has made significant progress in building international monitoring and disease surveillance capacity, which focuses on the current threat posted by the H5N1 avian influenza virus now circulating in birds in many parts of the world.
 HHS is actively engaged with States and with countries around the globe to help minimize and contain the impact of an influenza pandemic. Should such a pandemic occur, the international community will be better prepared than it was three years ago, due in large part to HHS funding and technical assistance that has contributed to stronger worldwide disease surveillance networks, improved laboratory capacity to analyze virus samples, and better trained workforce personnel.
HHS is actively engaged with States and with countries around the globe to help minimize and contain the impact of an influenza pandemic. Should such a pandemic occur, the international community will be better prepared than it was three years ago, due in large part to HHS funding and technical assistance that has contributed to stronger worldwide disease surveillance networks, improved laboratory capacity to analyze virus samples, and better trained workforce personnel.
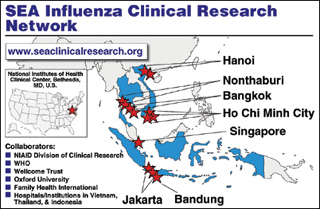
Through its own programs and in close coordination with other Federal agencies, HHS provides training, direct assistance, supplies, reagents, and technical support to the World Health Organization (WHO), to ministries of health, and to non-governmental organizations. Over the last three years, HHS has expanded its collaborative work, funding, and technical assistance to include countries across Asia, Africa, the Middle East, and Latin America. In August, HHS convened a meeting of scientists and public health professionals from 39 of those countries. The participants met to track progress and lay the groundwork for future preparedness and response activities.
HHS/CDC developed an inventory tool to help countries assess their levels of pandemic influenza preparedness and response. Over the last several months, CDC staff worked with leaders and technical experts in 42 countries to complete this inventory. The results will help evaluate current preparedness status across the globe and then measure progress against that baseline, toward increased preparedness over time.
 Rapid response funds primarily support avian influenza activities such as training for regional and national personnel, as well as exercises for mobile rapid response teams at six global disease detection centers around the world. These centers also maintain stockpiles of antivirals and personal protective equipment, build laboratory and epidemiological capacity, and direct on-the-ground surveillance programs and early warning systems.
Rapid response funds primarily support avian influenza activities such as training for regional and national personnel, as well as exercises for mobile rapid response teams at six global disease detection centers around the world. These centers also maintain stockpiles of antivirals and personal protective equipment, build laboratory and epidemiological capacity, and direct on-the-ground surveillance programs and early warning systems.
Teams of U.S. scientific experts join WHO teams to work with local scientists and public health officials and investigate suspected cases of human transmission of the H5N1 virus. To date, these teams have carried out their investigations in 12 countries in Asia, Africa, and Europe.
As part of its goal of building local capacity, HHS has led regional workshops to provide training for country-level rapid-response teams in 112 countries worldwide. As an outgrowth of this training, China stepped up its health monitoring in this year’s seasonal flu cycle and now has 198 monitoring hospitals and 63 laboratories reporting influenza cases on a weekly basis, as of October. This increased level of surveillance will help lead to a quicker identification of a pandemic influenza strain, should it appear in China.
The issue of virus sharing has been a contentious one for the past several years. HHS continues to hold firmly to the conviction that a strong global sample-sharing network is the best defense against an eventual pandemic. For sixty years, nations have openly and freely shared virus samples. It is one of the most important global health success stories.
top of page
“A pandemic is a health problem but it’s such a big health problem, it’s a societal continuity problem.”
– Dr. William Raub,
Science Advisor to the HHS Secretary
Over the past three years, HHS has invested more than $5.6 billion to help ensure that the Nation could effectively respond to an influenza pandemic. These funds focused on activities that could be undertaken immediately as well as those that will lead to longer-term outcomes.
Today many of the short-term goals have been met, and others are well on their way to producing positive interim results. Vaccine production has been expanded and diversified. Pre-pandemic vaccines and antivirals have been stockpiled throughout the country. Initial research into vaccine production based on cells rather than eggs has advanced, as has research on methods to help stretch the active component (antigen) of influenza vaccines.
New antiviral drugs are showing promise as they move through the testing pipeline. Development is well underway for diagnostic equipment that can test for influenza viruses at the patient’s bedside, an advance that will be of benefit for all influenza cases, not just in a flu pandemic. Similarly, medical supplies and ventilators are now stockpiled across the country.
While HHS has focused the majority of its direct efforts on vaccines and antiviral medications, it has also recognized that some decisions can only be made at the local level, such as how to dispense limited supplies of these vaccines and antivirals. HHS has directed additional resources to help local communities find common ground as they work together on this important task.
Pre-Pandemic Vaccine
 The development of the H5N1 pre-pandemic influenza vaccine is one of the signal achievements of the past three years’ efforts. This vaccine has now been tested and proven safe for people and represents the Nation’s first line of defense in the event of a pandemic.
The development of the H5N1 pre-pandemic influenza vaccine is one of the signal achievements of the past three years’ efforts. This vaccine has now been tested and proven safe for people and represents the Nation’s first line of defense in the event of a pandemic.
As of September 2008, HHS had purchased 12.2 million treatment courses of the pre-pandemic (H5N1) influenza vaccine. (A course is a cycle of treatment for one individual.) A small portion of this store of vaccines supports clinical trials now underway as well as those planned for the future. The rest is stockpiled and will be administered to protect healthcare workers, other first responders, and critical workers in the early stages of an influenza pandemic.
Vaccines
Video Clip

U.S. Public Health Service
Captain Ann Knebel, Office of the Assistant Secretary for Preparedness and Response/HHS.
A key goal of HHS pandemic planning is to provide pandemic influenza vaccine to every American within six months of the beginning of an influenza pandemic. To achieve this goal, HHS is investing in the expansion of vaccine manufacturing capacity (both egg- and cell-based), and the early development of next-generation recombinant vaccines.
Egg-Based Vaccines: The standard egg-based technology is essentially the same, whether producing seasonal or pandemic influenza vaccine. A robust seasonal flu production capacity could easily convert to production of a pandemic vaccine.
To help expand this standard production capacity, HHS awarded $120 million to vaccine manufacturers to retro-fit their existing U.S. vaccine manufacturing facilities. This allows for increases in the production of domestic egg-based seasonal, pre-pandemic and pandemic influenza vaccines. Manufacturing operations are being strengthened at new as well as converted facilities. Engineering and actual construction to renovate these existing facilities are ongoing.
A positive effect of this investment has been a substantial increase in the supply of seasonal flu vaccine. Manufacturers anticipate producing an all-time high of up to 146 million doses of seasonal influenza vaccine for the 2008-09 seasonal flu season.
Cell-Based Vaccines: Cell-based vaccines hold the potential to shorten the time between the identification of a pandemic virus and full-scale production of the vaccine for the U.S. population. In place of eggs, cell-based vaccine production uses laboratory-grown cell lines that can host a growing virus. Vaccine manufacturers can thus bypass the steps needed to adapt the virus strains so that they will grow in eggs. A vaccine can thus be produced in a matter of weeks.
In 2006, HHS invested more than $1 billion to support the advanced development of cell-based influenza vaccines. Contracts with six manufacturers are leading to the capacity for U.S.-based production of at least 240 million courses of cell-based pandemic vaccine within six months of the emergence of a pandemic influenza virus. To date, the five manufacturers are on target for reaching the milestones required in their contracts, and all are moving toward FDA approval of cell-based influenza vaccines.
Once the manufacturing process is approved, the facilities will also be able to produce cell-based vaccines for seasonal flu. And they will be able to adapt their production facilities to cell-based pandemic vaccines, when the threat of an influenza pandemic turns into reality.
In August 2008, HHS asked for proposals to build domestic cell-based vaccine production facilities. HHS will award approximately $500 million in contracts for this activity, in FY 2009. In the coming year, HHS expects to make several cost-sharing awards that will assist in the design, construction, commissioning and validation of new U.S. bulk manufacturing facilities for cell-based seasonal and pandemic influenza vaccines.
DNA-based Influenza Vaccines: DNA-based vaccines contain only portions of the influenza virus’ genetic material. Once inside the body, the DNA instructs human cells to make proteins that act as a vaccine against the virus.
By using the DNA of an avian influenza virus as the starting point to produce a vaccine, HHS hopes to offer the possibility of a vaccine with a shorter production timeline and quicker release of the vaccine to the public. In 2007, HHS issued a request for proposals for the preliminary development of this next generation of DNA-based influenza vaccines, leading to eventual U.S. licensure. Following that initial request for proposals, HHS is reviewing options for this recombinant DNA vaccine and plans to make awards once that review is complete. HHS is moving forward with plans to issue a second request for proposals for recombinant vaccine development.
Vaccine Allocation and Use
 Effective allocation of pandemic influenza vaccine will plan a critical role in preventing influenza and reducing its effects on health and society when a pandemic arrives.
Effective allocation of pandemic influenza vaccine will plan a critical role in preventing influenza and reducing its effects on health and society when a pandemic arrives.
In July, HHS and the U.S. Department of Homeland Security released guidance on allocating and targeting for pandemic influenza vaccine. The guidance provides a planning framework to help State, tribal, local and community leaders ensure effective and consistent vaccine allocation and use in the event of a pandemic.
The framework’s goal is to assist planners in reducing the public health impact of a pandemic, minimizing disruption to society and the economy, and enabling a speedier recovery from a pandemic. This guidance grew out of a deliberative democratic process that included consultations with individuals in communities, local and State leaders, public health specialists, and bioethicists. The guidance is available at http://www.pandemicflu.gov/vaccine/allocationguidance.pdf
Adjuvants
When adjuvants are successfully paired with influenza vaccines, the vaccines can be stretched to protect many more Americans and the vaccines may provide broader protection.
In 2005, HHS set a goal of providing pandemic influenza vaccine for 20 million people. Since then, planning efforts have also included identifying and testing adjuvants that could be combined with the pre-pandemic and pandemic vaccines to make those original 20 million available doses extend to protect more people.
Early research results have been promising and have pointed to the possibility of combining an H5N1 vaccine manufactured by one company with adjuvants from another company. The resulting “joint” vaccine could boost the effectiveness of each adjuvanted dose and lead to a far larger stockpile of vaccine. Further research is now underway to test this preliminary result, in what is known as a “mix-and-match” study of adjuvants among different vaccine manufacturers.
In the meantime, HHS will continue to purchase and stockpile additional doses of pre-pandemic vaccine. HHS is moving forward with the purchase of 5.2 million doses of adjuvant from GSK that will be paired with GSK antigen, currently held in bulk form in the HHS pre-pandemic vaccine stockpile.
Antiviral Drugs
A key goal of the HHS Pandemic Influenza Plan (2005) is to ensure that antiviral drug treatment courses are available for 25 percent of the U.S. population, with supplies on hand for initial containment efforts. The Federal government and States are working toward accomplishing this goal by together purchasing a total of 81 million courses of antiviral drugs.
In December 2007, HHS completed its goal of purchasing the 50 million courses of antiviral drugs (Tamiflu and Relenza) for the Federal stockpile. The majority of that stockpile (44 million courses) will be allocated proportionally to the States, territories, and the District of Columbia, based on population. The Federal government has reserved the remaining stockpiled six million treatment courses and expects to use them to help contain the spread of an emerging pandemic.
States have taken advantage of a Federal contractual subsidy that allows them to purchase a total of 31 million treatment courses of antiviral drugs through Federal best-price contracts and a 25 percent purchase subsidy. To date, the States have purchased 23 million treatment courses for their pandemic stockpiles, and 22 million of those courses have been delivered.
Over the past three years, manufacturers of the two antiviral drugs have increased their production capacity worldwide. With this increased availability, HHS now has the option of considering possibilities for expanding antiviral use within the population and could include in future planning such options as using antivirals as an added measure of prevention once a person is exposed to the virus or using them as a daily protective measure for those working on the front lines of a pandemic response.
Antiviral drug manufacturers are also working closely with private industry to have a fresh annual stockpile of antiviral drugs for their employees, to help ensure that the impact of a pandemic would not overwhelm local economies.
In addition to current antiviral drug options, HHS is also exploring the advanced development of a new class of influenza antiviral drugs that could be used during life-threatening cases of severe seasonal or pandemic influenza in hospitals. In January 2007, HHS awarded a contract for $102 million for the advanced development of such a new class of influenza antivirals. HHS is also funding research to investigate additional early-phase products that could also serve as antiviral candidates.
Antiviral Drug Allocation and Use During a Pandemic
In a similar process to the vaccine guidance, a Federal interagency working group drew on input from representatives of State, local and tribal public health agencies, and the public, as they considered scientific issues, ethics and values, and perspectives of stakeholders. The working group has developed a guidance document on antiviral use during an influenza pandemic. The guidance is available at http://pandemicflu.gov/vaccine/antiviral_use.pdf
While current antiviral drug use strategies and publicly maintained stockpiles are targeted primarily for treatment of persons with pandemic illness, expanded antiviral drug production has allowed additional strategies to be considered.
Proposed Considerations for Employer Antiviral Drug Stockpiling
 Private employers, too, are encouraged to plan and prepare for an influenza pandemic. In 2005, HHS issued a checklist of issues for employers to consider, as they developed their pandemic preparedness plans. Among the issues addressed was that of antiviral drug stockpiling. As a follow-up to that earlier checklist, HHS is planning to issue a series of considerations for employers who opt to stockpile antivirals in preparation for an influenza pandemic.
Private employers, too, are encouraged to plan and prepare for an influenza pandemic. In 2005, HHS issued a checklist of issues for employers to consider, as they developed their pandemic preparedness plans. Among the issues addressed was that of antiviral drug stockpiling. As a follow-up to that earlier checklist, HHS is planning to issue a series of considerations for employers who opt to stockpile antivirals in preparation for an influenza pandemic.
Broadly, the guidance recommends providing antivirals to those employees who are at very high risk of exposure to the virus for the duration of the community’s outbreak, as well as those who are critical to the community’s or the organization’s essential operations. In addition, some employers, who would have a lower risk of exposure, may consider providing antiviral medications for workers in order to maintain business continuity.
It should be noted that the guidance does not set a requirement or expectation that all employers should stockpile antiviral drugs, nor does the guidance create a standard-of-care requirement. Rather, it defines a prudent strategy for employer antiviral drug stockpiling and use that can contribute to a more effective pandemic response. The guidance is available at http://pandemicflu.gov/vaccine/antiviral_employers.pdf.
Diagnostics
Diagnostic tools, whether used in a laboratory or at a patient’s bedside, help identify a disease and thus shorten the time to the application of the correct treatment. HHS has awarded funds to developers of new point-of-care influenza rapid test devices that will help doctors and other health professionals distinguish between seasonal and pandemic flu rapidly and easily in a clinic, office or other outpatient setting.
Clinical trials of these rapid test devices are also anticipated in the near future. Manufacturers hope to submit these devices to FDA for approval by the end of 2009.
In addition, FDA has recently approved a new highly sensitive rapid diagnostic test that was developed by CDC to detect and identify common human flu viruses as well as the avian flu virus. This test will be available to all U.S. public health laboratories and internationally at WHO reference laboratories. It will serve as the gold standard for differentiating between seasonal flu and the potential first cases of pandemic influenza.
HHS plans to continue supporting the use of simple influenza tests for detecting any influenza virus. Such tests would allow for greater testing capabilities during a pandemic and would help communities and individuals in their pandemic decision-making.
Other laboratory influenza and point-of-care tests are also in development. Current rapid tests could be adapted for use in non-healthcare settings, along with improved and more reliable methods for collecting specimens for testing. HHS expects to award contracts for improved specimen collection by the end of this year.
Medical Supplies
Personal protective equipment, such as surgical masks and respirators, helps reduce exposure to viruses and would thus help reduce the spread of the disease during an influenza pandemic.
HHS has purchased 158 million masks and respirators and has obligated $100 million to purchase ventilators, syringes, and intravenous antibiotics for potential distribution in case of an influenza pandemic, along with needles, gloves, surgical gowns and other supplies. Stockpiled ventilators are intended to provide breathing assistance to critically ill patients.
HHS has developed a common-sense guidance on use of masks and respirators for individuals and families, to learn how best to use this simple, everyday protection. HHS requested and received comments from the public in June about this common-sense guidance and expects to release the final version next year.
Other Research
HHS has also supported research to study patterns of disease from the past century’s pandemics and influenza epidemics to recognize early warning signals of pandemics and to help prioritize control efforts. Historical data has demonstrated that pandemics occur in waves and affect different age groups related to presumed historic exposures to past flu viruses. HHS has supported workshops with collaborators from over 40 countries to study how influenza virus during epidemic and pandemic years impacts mortality and how waves of illnesses move through temperate and tropical regions.
By studying the 1918 pandemic, which affected millions of people across the globe, researchers learned that those who lived through that 1918 pandemic outbreak and were alive today could still produce antibodies that killed the deadly strain of the H1N1 flu, ninety years later. Results of this study could lead to the development of emergency treatments for future outbreaks.
Other researchers learned that the majority of deaths during the influenza pandemic of 1918-1919 were not caused by the influenza virus acting alone. Rather, the deaths likely resulted from bacterial pneumonia and related infections that took advantage of the victims’ weakened immune systems.
While it has long been recognized that most seasonal flu deaths are due to pneumonia caused by secondary bacterial infections, the results of a new study have further stimulated planners to consider antibiotics or bacterial vaccines, which combat such infections, to the arsenal of pandemic countermeasures.
top of page
“We work with our counterparts in Mexico and Canada. We share data. We make sure the labs can define the virus. We share knowledge about surveillance. ”
– Admiral Joxel Garcia,
HHS Assistant Secretary for Health
States, cities and local communities have been the cornerstone of preparedness since the earliest days of pandemic planning. In 2005-06, HHS conducted a pandemic influenza summit in every State and territory. These summits brought HHS leaders together with State leadership, health professionals, schools, and the private sector. Together they have forged partnerships that laid the foundation for advances in State and community preparedness.
HHS awarded $576 million to States and local communities, to build their pandemic influenza preparedness networks, including medical surge capacity. This funding focused on practical, community-based procedures that could prevent or delay the spread of an influenza pandemic. In September, HHS awarded an additional $24 million to fund projects in 29 State and local health departments that could serve as innovative approaches to influenza pandemic preparedness.
HHS continues to contribute to advancing the thinking in healthcare system surge capacity and preparedness levels during a pandemic. In July, HHS released a report – Home Health Care During an Influenza Pandemic: Issues and Resources – that explores the key issues and challenges in providing home healthcare services during a flu pandemic. The report is available at http://www.pandemicflu.gov/plan/healthcare/homehealth.html.
Planning and Exercises
 Effective State, local and community response during and after an influenza pandemic requires two critical steps before the pandemic begins – planning and practice. Both key steps help ensure that States and local governments can maintain their critical functions as the impact of a pandemic is felt throughout the country.
Effective State, local and community response during and after an influenza pandemic requires two critical steps before the pandemic begins – planning and practice. Both key steps help ensure that States and local governments can maintain their critical functions as the impact of a pandemic is felt throughout the country.
HHS has provided guidance to States and local communities to develop and update their pandemic influenza plans and to ensure that they evaluate their plans through exercises, or simulated emergencies. In this way the plans can be tested in real time and adjusted as needed. To encourage States and communities to update and test their plans, HHS provided approximately $150 million for that purpose, as part of the September 2006 funding for the State plans.
In 2007, HHS made available $75 million to States, territories and four metropolitan areas to help strengthen their capacity to respond to a pandemic influenza outbreak. Through this funding, the governments are working to establish stockpiles of critical medical equipment and supplies, as well as develop plans for maintenance, distribution, and sharing of resources at the State and sub-State levels.
In February 2007, HHS developed and distributed guidance to States and communities on the use of community mitigation strategies. To expand on this guidance, in March 2008 HHS provided Federal guidance to assist States in improving their pandemic influenza operating plans and supported that guidance with web-based seminars to provide technical assistance to States, as they developed their preparedness and response plans. (See PlanFirst Webcast Series, below.)
Public Education
PlanFirst Webcast Series
 In March, HHS launched PlanFirst, a regular webcast series of live, internet dialogues engaging Federal and State officials and community members on pandemic planning. These webcasts aim to help States, local communities, employers, schools, faith-based and civic organizations, and families and individuals learn more about pandemic planning. Program topics have included individual preparedness, home healthcare agency planning, workplace preparation, and the State planning process.
In March, HHS launched PlanFirst, a regular webcast series of live, internet dialogues engaging Federal and State officials and community members on pandemic planning. These webcasts aim to help States, local communities, employers, schools, faith-based and civic organizations, and families and individuals learn more about pandemic planning. Program topics have included individual preparedness, home healthcare agency planning, workplace preparation, and the State planning process.
“It’s not enough just to have written a plan…you also have to check it, test it and make sure that it works.”
– Dr. David Nabarro,
United Nations Influenza Coordinator
In October, HHS Secretary Mike Leavitt led a webcast featuring a panel of government and public specialists. The hour-long broadcast focused on pandemic planning and preparedness.
To date, HHS has broadcast seven episodes of PlanFirst. Guest presenters have included the American Red Cross, Johns Hopkins University School of Public Health, the U.S. Departments of Veterans Affairs, Labor, Transportation, Homeland Security, and Education, as well as the National Guard and the Reuters news agency.
The live Internet audience doubled with each program, reaching a high of more than 1,000 viewers for the October program featuring the HHS Secretary. Each program in the series has been downloaded for viewing more than 10,000 times.
Future programs will focus on vaccine research and development and community-level planning. Information about past programs, including archived copies of each program, can be found at http://www.pandemicflu.gov/news/panflu_webinar.html.
1918 Storybook
 To highlight the significance of the 1918 pandemic and the toll it took, a collection of personal stories is now available online, highlighting narratives from survivors, families, and friends who lived through the 1918 and 1957 pandemics. These eloquent stories from past pandemics serve as a sobering reminder of the devastating impact that influenza can have. Reading them puts a human face on today’s public health preparedness activities. The Storybook is available at http://www.pandemicflu.gov/storybook/index.html.
To highlight the significance of the 1918 pandemic and the toll it took, a collection of personal stories is now available online, highlighting narratives from survivors, families, and friends who lived through the 1918 and 1957 pandemics. These eloquent stories from past pandemics serve as a sobering reminder of the devastating impact that influenza can have. Reading them puts a human face on today’s public health preparedness activities. The Storybook is available at http://www.pandemicflu.gov/storybook/index.html.
Take the Lead: Working Together to Prepare Now
Government alone cannot prepare the Nation for pandemic flu. This challenge requires everyone’s help. Leaders in local communities play a powerful role in encouraging their employees, patients, members, and others to prepare for a pandemic.
“It’s not going to be a Federal response as much as it’s going to be a local response. So every community has to be prepared. ”
– Mayor Tim Woerther, Wildwood MO
Through its Take the Lead series of information materials and suggested activities, HHS encouraged leaders in nine pilot communities across the country, to bring the preparedness message to those in their spheres of influence. The success and lessons learned from this experience are now being woven into the next phase of the campaign.
National Preparedness Month
As part of its Take the Lead campaign, HHS encouraged its national partners and pilot sites to engage in simple activities to promote the issue of pandemic preparedness during National Preparedness Month, September, sponsored by the U.S. Department of Homeland Security. Local activities included special events, media outreach, and distribution of planning materials.
Vulnerable Population Planning
Those within a community who have fewer resources to draw on are often more likely to experience a disproportionate impact of a pandemic. To help HHS, States and communities address these populations more effectively, before, during, and after a pandemic, research is now underway to test pandemic influenza messages on a variety of vulnerable and at-risk populations. This work will analyze health literacy and the flow of communications from public health sources to these groups within the community.
The research includes analysis of readiness for action of vulnerable populations in response to a pandemic, and new methods to improve the ways messages are prepared that help make them more understandable to a broader audience.
HHS has also partnered with the Association of State and Territorial Health Officials (ASTHO) to develop guidance for State, territorial tribal, and local health officials on how to protect at-risk populations during an influenza pandemic. Nearly all States have received training on this topic through regional workshops and webinars.
top of page
2004 – H5N1 vaccine reference created by reverse genetics from H5N1 virus isolated in Vietnam
April 2005 – RFP for cell-based vaccine contract issued
November 2005 – President Bush announces the National Strategy for Pandemic Influenza
Summer 2005 – Preliminary results form H5N1 vaccine clinical trials indicate immune response predictive of protection against H5N1
September 2005 – International Partnership on Avian and Pandemic Influenza launched
November 2005 – HHS launches Pandemic Influenza Plan
December 2005 – Passage of the Public Readiness and Emergency Preparedness Act (PREP Act)
December 2005 – First state summit in MN
January 2006 – RFI issued on increasing egg-based vaccine capacity
January 2006 – 6 State summits in AZ, VT, WV, RI, GA, KY
January 2006 – RFI issued on advanced development of promising antivirals
January 2006 – Release of individuals and families, faith-based and community organizations, and school districts (K-12) checklists
February 2006 – State summits in CT, IA,A, FL, OH, NV, DE, AL, MO, NE, MD
February 2006 – Laboratory assay for diagnostic testing of avian influenza A/H5 approved
March 2006 – Releases of medical offices and clinics, home health care, child care and preschool, and colleges and universities checklists
March 2006 – Pandemic flu supplemental guidance sent to States
March 2006 – 19 state summits in SC, SD, ND, WY, WI, PA, IL, NC, VA, IN, PR, CO, UT, ID, TX, NM, OR, CA, and the Virgin Islands
March 2006 – RFP issued for advanced development of antigen-sparing technologies
March 2006 – FDA approves Relenza for the prevention of Influenza A and B in adults and children
March 2006 – States receive initial grant funding for pandemic preparedness
March 2006 – Order of 16.2 million courses of Tamiflu and 3.9 million courses of Relenza
April 2006 – State summits in TN, AK, WA, HI, LA, MI and the District of Columbia
April 2006 – FDA issues guidance for industry on diagnostic devices to detect influenza A viruses
May 2006 – Homeland Security Council releases National Strategy for Pandemic Influenza: Implementation Plan
May 2006 – HHS awards contracts totaling more than $1 billion to develop cell-based influenza vaccine
May 2006 – Department of State establishes Avian Influenza Action Group to coordinate U.S. international effort
May 2006 – Release of long-term care and other residential checklist
May 2006 – 7 State summits in MS, OK, NJ, KS, NH, MT and tribal leaders
June 2006 – CDC issues updated interim guidance for laboratory testing of persons with suspected infection with avian influenza A (H5N1) virus in the United States
June 2006 – Congress passes $2.3 billion funding for pandemic preparedness
July 2006 – Summits in AK and New York City
July 2006 – HHS allocates $170 million to subsidize State purchase of antiviral drugs
August 2006 – State summit in NY
September 2006 – FDA issues guidance to industry for development of cell-based viral vaccines
October 2006 – Guidance on use of surgical masks and respirators in health care settings released
November 2006 – HHS awards $199 million to manufacturers for 5.3 million doses of H5N1 vaccine
December 2006 – NIH begins human clinical trials of DNA-based H5N1 vaccine
January 2007 – HHS awards $132.5 million to develop H5N1 adjuvanted influenza vaccines
January 2007 – HHS awards $102.6 million for advanced development of new influenza antiviral drug, peramivir
January 2007 – U.S. businesses with overseas operations checklist released
February 2007 – HHS launches pandemic flu public service announcements
February 2007 – CDC releases community mitigation guidance
February 2007 – 2,250 human and avian influenza virus genomes completed and publicly accessible
March 2007 – Health insurers planning checklist released
April 2007 – FDA approves first U.S. H5N1 human influenza vaccine
April 2007 – 12 million doses of human H5N1 vaccine added to Strategic National Stockpile
April 2007 – Travel industry planning checklist released
April 2007 – NIH awards $161 million to expand surveillance and bolster research
May 2007 – HHS issues interim guidance on community (nonwork) use of facemasks
May 2007 – FDA clears first respirators for public health emergencies
May 2007 – New online training course released for State and local public health responders
June 2007 – Two contracts awarded to expand domestic vaccine manufacturing capacity
June 2007 – Pandemic Flu Leadership Forum
July 2007 – HHS announces $896.7 million in funding to states for public health preparedness and emergency response
July 2007 – Homeland Security issues one-year summary on the National Strategy Implementation Plan
August 2007 – North American plan for avian and pandemic influenza released
August 2007 – $75 million released to states to strengthen pandemic response capacity
September 2007 – Planning checklists released for law enforcement and correctional facilities
December 2007 – 8 suspected human cases of H5N1 in Pakistan
December 2007 – Myanmar confirms first human H5N1 infection
December 2007 – 3-day web dialogue on vaccine allocation
December 2007 – first human case confirmed in Pakistan
December 2007 – HHS launches “The Great Pandemic” historical overview of 1918 pandemic
December 2007 – HHS launches “Take the Lead: Working Together to Prepare Now” campaign
February 2008 – EU FDA-counterpart gives preliminary approval to pre-pandemic vaccine
March 2008 – HHS holds first of 3 state pandemic planning webinars
May 2008 – Bangladesh confirms its first case of human infection with H5N1 avian influenza
June 2008 – CDC awards $12.5 million in contracts to develop faster influenza diagnostic tests
July 2008 – AHRQ issues report on home health care during an influenza pandemic, covering issues and resources
July 2008 – HHS and DHS announce guidance on pandemic vaccination allocation
September 2008 – CDC awards $24 million for innovative pandemic influenza preparedness projects
September 2008 – FDA clears new CDC test to detect human influenza
October 2008 – CDC awards $16.9 million contract to improve capabilities to combat pandemic, seasonal influenza
October 2008 – U.S. commits additional $320 million for avian and pandemic influenza assistance; U.S. international support now totals $949 million.
October 2008 – HHS Secretary Leavitt hosts pandemic web discussion on pandemic planning and preparedness
November 2008 – U.S. pledges additional $44.4 million to UN avian influenza control and preparedness campaign.
top of page