|
Issue
#11 - Fall 2006
FDA Approves New Products for Cervical Cancer
Prevention and Detection
 FDA recently announced the approval of two new products that will help prevent and detect cervical cancer. Gardasil is the first vaccine developed to prevent cervical cancer, precancerous genital lesions and genital warts due to human papillomavirus (HPV) in females 9-26 years of age. FDA has also approved the LUMA Cervical Imaging System that can help detect the signs of cervical cancer in its most treatable stage. FDA recently announced the approval of two new products that will help prevent and detect cervical cancer. Gardasil is the first vaccine developed to prevent cervical cancer, precancerous genital lesions and genital warts due to human papillomavirus (HPV) in females 9-26 years of age. FDA has also approved the LUMA Cervical Imaging System that can help detect the signs of cervical cancer in its most treatable stage.
HPV is the most common sexually-transmitted infection in the U.S. The Centers for Disease Control and Prevention estimates that about 6.2 million Americans become infected with genital HPV each year and that over half of all sexually active men and women become infected at some time in their lives. On average, there are 9,710 new cases of cervical cancer and 3,700 deaths attributed to it in the U.S. each year. Worldwide, cervical cancer is the second most common cancer in women; and is estimated to cause over 470,000 new cases and 233,000 deaths each year.
For most women, the body's own defense system will clear the virus and infected women don't develop related health problems. However, some HPV types can cause abnormal cells on the lining of the cervix that years later can turn into cancer. Other HPV types can cause genital warts. The vaccine is effective against HPV types 16 and 18, which cause approximately 70 percent of cervical cancers and against HPV types 6 and 11, which cause approximately 90 percent of genital warts.
The New Vaccine
Gardasil contains no live virus, and is given as three injections over a six-month period. Immunization with Gardasil is expected to prevent most cases of cervical cancer due to HPV types included in the vaccine. However, females are not protected if they have been infected with that HPV type(s) prior to vaccination. It is important to immunize before potential exposure to the virus. Also, Gardasil doesn't protect against less common HPV types not included in the vaccine, so routine and regular Pap screening remain critically important to detect precancerous changes and allow treatment before cervical cancer develops.
Four studies, one in the U.S. and three multinational, were conducted in 21,000 women to show how well Gardasil worked in women between the ages of 16 and 26 by giving them either the vaccine or placebo. The results showed that in women who had not already been infected, Gardasil was nearly 100 percent effective in preventing precancerous cervical lesions, precancerous vaginal and vulvar lesions, and genital warts caused by infection with the HPV types against which the vaccine is directed. While the study period was not long enough for cervical cancer to develop, the prevention of these cervical precancerous lesions is believed highly likely to result in the prevention of those cancers.
The studies also evaluated whether the vaccine can protect women already infected with some HPV types included in the vaccine from developing diseases related to those viruses. The results show that the vaccine is only effective when given prior to infection.
Two studies were also performed to measure the immune response to the vaccine among younger females aged 9-15 years. Their immune response was as good as that found in 16-26 year olds, indicating that the vaccine should have similar effectiveness when used in the 9-15 year age group.
The safety of the vaccine was evaluated in approximately 11,000 individuals. Most adverse experiences included mild or moderate local reactions, such as pain or tenderness at the site of injection.
The manufacturer will continue to conduct several studies, including additional studies to further evaluate general safety and long-term effectiveness. It will also monitor the pregnancy outcomes of women who receive Gardasil while unknowingly pregnant and will continue to evaluate the safety and effectiveness of Gardasil in males.
The New Imaging System
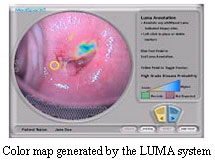 FDA has also approved the LUMA Cervical Imaging System (LUMA). LUMA can help detect a cervical cancer precursor, a sign of possible cancer development, by identifying sites on the cervix that may contain pre-cancerous cells. It is intended to be used along with colposcopy, a high magnification evaluation of the cervix for women who have recently had an abnormal Pap test. In the manufacturer’s study of 193 women, the LUMA image helped the doctor detect additional cancer precursors missed by colposcopy. Of the 50 cases of pre-cancer detected in the study, colposcopy caught 41 and LUMA caught an additional 9 cases that colposcopy had missed. This additional yield of 9 true-positive cases came at the cost of approximately one additional biopsy per patient. FDA has also approved the LUMA Cervical Imaging System (LUMA). LUMA can help detect a cervical cancer precursor, a sign of possible cancer development, by identifying sites on the cervix that may contain pre-cancerous cells. It is intended to be used along with colposcopy, a high magnification evaluation of the cervix for women who have recently had an abnormal Pap test. In the manufacturer’s study of 193 women, the LUMA image helped the doctor detect additional cancer precursors missed by colposcopy. Of the 50 cases of pre-cancer detected in the study, colposcopy caught 41 and LUMA caught an additional 9 cases that colposcopy had missed. This additional yield of 9 true-positive cases came at the cost of approximately one additional biopsy per patient.
LUMA shines a light on the cervix and analyzes how different areas respond to the light. LUMA assigns a score to tiny areas of the cervix and produces a color map that helps a doctor decide where to biopsy. The colors and patterns on the map help the doctor distinguish between healthy tissue, and potentially diseased tissue.
First a doctor performs a colposcopy and identifies areas on the cervix to biopsy. Then the doctor evaluates the LUMA image to see whether or not there are additional areas of the cervix that should be biopsied. Only after both the colposcopy and LUMA procedures are completed does the doctor perform biopsies.
FDA's approval was based on data from the firm's clinical study of 193 women who underwent colposcopy, followed by LUMA. FDA's analysis showed that the device is safe and effective and that -- when used along with colposcopy -- LUMA will help detect additional cervical cancer precursors.
For more information visit:
Sources: FDA Licenses New Vaccine for Prevention of Cervical Cancerand Other Diseases in Females Caused by Human Papillomavirus, FDA Approves New Imaging System to Help Detect Cervical Pre-Cancer
Back to Table of Contents
Editors’ Note: This month FDA & YOU is introducing a new column, Medicines In My Home, which will address the safe and effective use of over-the-counter medicines.
Medicines In My Home:
What do you know about over-the-counter (OTC) medicines?
The RULES
for Using Medicines Safely
Do
- Speak to your parent or guardian before using any medicine.
- Read the Drug Facts panel on the label - ALL of it - and follow the directions.
- Check ingredients. This is especially important if you are using more than one medicine. Make sure you are not using two medicines with the same active ingredient.
- Choose a medicine that treats only the problems you have.
- Tell your parent, guardian, or school nurse if you do not feel better or start to feel worse after using a medicine.
- Speak to your doctor, nurse, or pharmacist if you have questions about your medicine or how it should make you feel.
- Keep all medicines in the bottle, box, or tube that they came in. That will make the directions easy to find.
- Keep all medicines in a safe, dry place. Keep them where they can't be seen or reached by younger children or pets.
Don't
- Don’t use a medicine unless you know what it is and what it’s for.
- Don’t use more medicine than the amount listed on the label. If the medicine does not help you feel better, tell an adult. Don't take more of the medicine.
- Don’t use other people's prescription medicine and don’t share your prescription medicine with anyone else.
- Don’t take medicine for longer than the label says.
- Don’t use old medicines. If a medicine is past the expiration date on the package, throw it in a garbage can away from small children and pets. This is the best choice if you are unable to take medicines to a household hazardous waste site.
|
Have you ever used an over-the-counter (OTC) medicine? People use OTC medicines every day - sometimes without even realizing it. Most people know that pain relievers, antihistamines to treat allergies, and cough syrups are medicines, but many don’t realize that fluoride toothpastes, antiperspirants, and sunscreens are also OTC medicines.
A medicine either changes the way your body works, or treats or prevents a disease or problem. OTC medicines are the kind you can buy without a prescription (or order) from your doctor. Just like learning to look both ways before crossing the street and to fasten your seat belt when you get in a car, learning how to use OTC medicines safely is an important part of growing up healthy.
All OTC medicines have a special label on the package called Drug Facts . Reading Drug Facts is very important before using any OTC medicine.
Drug Facts tells you:
- The active ingredients (the parts of the medicine that make it work)
- What the medicine treats (its job or purpose)
- If the medicine is right for you and your problem
- How to use the medicine correctly (how much to use, how often to use it, when to speak to a doctor before using it, and when to stop using it).
It is important to use OTC medicines safely. Even though you can buy OTC medicines off the shelf in the store, these medicines can be harmful if you don’t use them carefully and correctly. So, ask questions! Play an active part in making choices about your health:
- Know what your medicine does and why you’re using it.
- Know what kinds of unwanted effects to watch for.
To learn more about the safe use of OTC medicines, please visit the Medicine in My Home (MIMH) Web site at http://www.fda.gov/medsinmyhome.
To sign up for email notices of new additions to the MIMH site visit https://list.nih.gov/archives/medicines-in-my-home.html
Back to Table of Contents
Improved Food Label May Help People with Food Allergies
When you drink a glass of milk, are you consuming casein and lactoglobulin? How about potassium caseinate and lactalbumin?
According to food scientists, all of these substances are proteins found in milk. And until recently, food manufacturers could use technical terms such as these on food labels without further explanation, instead of listing the more common term "milk."
That changed at the beginning of 2006, when a new food labeling law, the Food Allergen Labeling and Consumer Protection Act (FALCPA), took effect. The new label may help people with food allergies avoid products that could cause severe or life-threatening allergic reactions.
Did You Know?
About 2 percent of adults and 5 percent of infants and young children in the U.S. suffer from food allergies. About 30,000 consumers require emergency room treatment, and 150 Americans die each year because of allergic reactions to food.
There is no cure for food allergies. Avoiding food that causes the allergy is the only way to prevent a reaction. The improved label will make it easier for people to identify and avoid foods that contain major food allergens. For example, if a product contains the milk-derived protein casein, the product's label must state "milk" in addition to "casein" so those with milk allergies can clearly understand the presence of the allergen they need to avoid.
|
Under FALCPA, food labels are required to state clearly whether the food contains a major food allergen. These allergens account for 90% of all food allergies in the U.S. The law identifies as a major food allergen any of eight allergenic foods:
- milk
- eggs
- fish (such as bass, flounder, and cod)
- crustacean shellfish (such as crab, lobster, and shrimp)
- tree nuts (such as almonds, walnuts, and pecans)
- peanuts
- wheat
- soybeans
The law also identifies as a major food allergen any ingredient that contains protein derived from any of these eight foods. The new law also requires manufacturers to list flavorings, colorings, and incidental additives that are or contain a major food allergen.
New Label Requirements
Manufacturers must identify the presence of a major food allergen in one of two ways
- In the list of ingredients, manufacturers must state the source of an allergenic ingredient in parentheses after the name of that ingredient
Ingredients: Enriched flour (wheat flour, malted barley, niacin, reduced iron, thiamin mononitrate, riboflavin, folic acid), sugar, partially hydrogenated soybean oil, and/or cottonseed oil, high fructose corn syrup, whey (milk), eggs, vanilla, natural and artificial flavoring, salt, leavening (sodium acid pyrophosphate, monocalcium phosphate), lecithin (soy), mono- and diglycerides (emulsifier)
- After or next to the ingredient list, manufacturers must add "contains" followed by the name of the source of each allergenic ingredient in the food.
Ingredients: Enriched flour, sugar, partially hydrogenated soybean oil, and/or cottonseed oil, high fructose corn syrup, whey, eggs, vanilla, natural and artificial flavoring, salt, leavening, lecithin, mono- and diglycerides
Contains: Wheat, Milk, Eggs and Soy
These statements are not required if the major food allergen's common name already identifies its food source. For example, the ingredients whole wheat flour, buttermilk, and peanut butter already state that they contain wheat, milk, and peanuts, respectively, so no further explanatory terms are required.
FALCPA applies to both domestically produced and imported packaged foods FDA regulates. So even if you purchase a box of candy from overseas, it should have the correct label. Raw agricultural products, such as fresh fruits and vegetables, are exempt from FALCPA. So are highly refined oils made from one of the eight allergenic foods identified by the law.
The law applies only to products labeled on or after January 1, 2006, so until products labeled before that date are sold or otherwise removed from the market, consumers will continue to see some products without the plain language allergen labeling on grocery store shelves, particularly non-perishables such as canned goods.
FDA will enforce the new law and will document violations of the law when inspecting manufacturing plants. A food product that contains an undeclared allergen may be subject to recall. In addition, a food product not properly labeled may be misbranded and subject to seizure and removal from the marketplace.
Advice to Parents
Did You Know?
Even if consumers have purchased a product in the past that doesn’t aggravate their allergies, they shouldn’t assume it will always be nonallergenic. FDA encourages consumers to always read the ingredients list, because manufacturers may change their product formulation.
|
Food allergies are on the rise in children and improved food labeling information is especially helpful to those who must learn to recognize the presence of substances they need to avoid. Parents can help teach young children to start reading food labels while grocery shopping. Have children read the label and figure out if the product contains an allergy-causing food and whether it should go in the grocery cart or go back on the shelf.
If the child is too young to read, read the label to the child. It's almost impossible to keep the attention of a 5-year-old when you say 'ammonium caseinate,' but when you say 'milk,' the child with a milk allergy will think, 'That's not good for me.'
For More Information
Visit FDA's Food Allergens Page at
http://www.cfsan.fda.gov/~dms/wh-alrgy.html
Source: FDA Consumer
Back to Table of Contents
 What’s the difference between a cosmetic and an What’s the difference between a cosmetic and an
over-the-counter (OTC) drug product?
According to the Federal Food, Drug, and Cosmetic Act, cosmetics are products intended to be applied to the face or body for
- cleansing
- beautifying
- promoting attractiveness
- altering the appearance
Cosmetics may also be used inside the body. Examples include some mouthwashes, toothpastes, and douches. Cosmetics are not intended to affect the body's structure or functions.
Drugs are products intended to
- Diagnose, cure, lessen, treat, or prevent disease, or
- Affect the structure or any function of the body
This definition of a drug excludes food. Over-the-counter (OTC) or nonprescription drugs are drug products that can be bought without a doctor's prescription.
Some products meet the definitions of both cosmetics and drugs. This may happen when a product has two intended uses. For example, a shampoo is a cosmetic because it is intended to cleanse the hair. An antidandruff treatment is a drug because its intended use is to treat dandruff. As a result, an antidandruff shampoo is both a cosmetic and a drug. Other cosmetic/drug combinations include: toothpastes that contain fluoride, deodorants with an antiperspirant, medicated douches, and moisturizer and makeup marketed with sun protection claims.
The legal difference between a cosmetic and a drug is determined by a product's intended use. Different parts of the law and FDA regulations apply to each type of product. The drug parts of the law and FDA regulations apply to products that are both a cosmetic and a drug.
Want to know more? Visit http://www.cfsan.fda.gov/~dms/cos-218.html
Source: CFSAN Office of Cosmetics and Colors Web page
Back to Table of Contents
 Learn About It Online: Learn About It Online:
Medicines In My Home
FDA's Center for Drug Evaluation and Research (CDER), in cooperation with Maryland's Montgomery County Public Schools and the National Council on Patient Information and Education (NCPIE), has launched Medicines in My Home, an interactive educational program about the safe and effective use of over-the-counter medicines. The website provides classroom materials and resources for teachers and online information for students and their families.
The program focuses on understanding components of the Drug Facts label. It also emphasizes that medicines should be used only with adult supervision and advises that a doctor or pharmacist is the best source to answer questions about medicines. Some of the printable materials are designed to encourage students to share what they learn with their families so that all family members can learn to use over-the-counter medicines more safely.
Back to Table of Contents
 FDA Approves Influenza Vaccines for Upcoming Flu Season FDA Approves Influenza Vaccines for Upcoming Flu Season
FDA has approved this year's seasonal influenza (flu) vaccines that include the new strains of virus judged likely to cause flu in the U.S. in 2006-2007.
Each year flu vaccine manufacturers submit information and samples to FDA of their virus strains for the upcoming flu season for review and testing in FDA laboratories. Because different flu virus strains may appear each year, one or more of the strains in the vaccine may need to be changed to protect against what public health experts think are the strains most likely to infect people that year. This season's approved formulation for the U.S. vaccine is identical to that recommended by both the World Health Organization and FDA's Advisory Committee. The formulation includes one strain that was used in last year's vaccine and two new strains. Seasonal flu vaccines don’t protect against avian flu, which is caused by different viral strains.
Influenza or "the flu" is a contagious respiratory illness caused by flu viruses. It can cause mild to severe illness, and at times can lead to death. The best way to prevent the flu is to get vaccinated. Although it’s best to be immunized in the fall, getting the vaccine in the winter months when flu season often peaks is also recommended.
According to the CDC, every year in the U.S., on average five to 20 percent of the population gets the flu, more than 200,000 people are hospitalized from its complications, and approximately 36,000 people die from it. Some people are at high risk for serious complications from flu, such as the elderly, children, and people with certain chronic medical conditions.
Source: FDA Press Release http://www.fda.gov/bbs/topics/NEWS/2006/NEW01423.html
Back to Table of Contents
Brushing Up On Halloween Safety
 FDA's Halloween safety Web site is packed with simple tips to take the guess work out of planning a safe and healthy holiday, so you can concentrate on having a ghoulishly good time. FDA's Halloween safety Web site is packed with simple tips to take the guess work out of planning a safe and healthy holiday, so you can concentrate on having a ghoulishly good time.
The Web site includes FDA information on
- Treats
- Makeup
- Novelty Contact Lenses
- Safe costume and trick-or-treating (from the Consumer Product Safety Commission)
Back to Table of Contents
Online Tool Uses Family Health History to Predict Disease
 A free online tool is available to help families gather their health information. A free online tool is available to help families gather their health information.
"I encourage all families to take time to collect important health history information that can benefit all family members," then U.S. Surgeon General Richard H. Carmona, M.D., M.P.H., said in announcing the new tool in February 2006. "Even with all the high-tech tests, medicines, and procedures available in today's modern health-care setting, family health history remains the cornerstone of our efforts to prevent disease and promote personal health. It's clear that knowing your family history can save your life."
Health care professionals have known for a long time that many diseases, such as cancer, diabetes, and heart disease, can run in families. A detailed family history can predict the disorders for which a person may be at increased risk and can help develop more personalized approaches to prevent illness.
"My Family Health Portrait," can be operated on computers with Internet access running any one of several standard browsers. The tool organizes a family's health history into a printout that people can take to their health care professional to help determine whether they are at higher risk for disease. All personal information entered into the program remains private and is saved on the user's computer only.
The tool guides users to compile information for their family members on six common diseases--heart disease, stroke, diabetes, colon cancer, breast cancer, and ovarian cancer--as well as information about any other conditions that are of particular interest to the family. The tool focuses on these six diseases because a genetic link is known for each and because a preventive strategy can be developed to avoid illness.
"My Family Health Portrait" is part of a national public health campaign called the U.S. Surgeon General's Family History Initiative, which encourages all American families to learn more about their family health history. The surgeon general launched the campaign in cooperation with other organizations within the U.S. Department of Health and Human Services (HHS), including the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health (NIH); the Centers for Disease Control and Prevention (CDC); the Agency for Healthcare Research and Quality (AHRQ); and the Health Resources and Services Administration (HRSA).
English: https://familyhistory.hhs.gov/
Spanish: https://familyhistory.hhs.gov/spanish
U.S. Surgeon General's Family History Initiative: http://www.hhs.gov/familyhistory
Source: FDA Consumer
Back to Table of Contents
 A Guide to FDA Recalls A Guide to FDA Recalls
Recalls are a way to remove or correct products that violate laws enforced by FDA. Once a problem or a potential problem is discovered, a company initiates a product recall in cooperation with FDA. Most recalls of products regulated by the FDA are done voluntarily by the manufacturer.
The most efficient and effective way to get defective products off the market quickly and protect the public health is voluntary manufacturer recalls. This avoids court actions which can be time-consuming and costly.
FDA oversees recalls for all products it regulates
- Human drugs
- Medical devices and radiation-emitting products such as x-ray equipment, TVs and microwaves
- Biologics such as vaccines and blood products
- Veterinary products, including animal drugs and animal feed
- Cosmetics
- About 80 percent of the foods consumed in the U.S. The Food Safety and Inspection Service, part of the U.S. Department of Agriculture (USDA), handles recalls for meat, poultry, and certain egg products.
Types of Voluntary Recalls
Sometimes, a company discovers a problem with a product and then contacts FDA. That's what happened in a highly unusual recall announced in February 2006 by Mead Johnson for a batch of its 24-ounce cans of GENTLEASE powdered infant formula.
Mead Johnson informed FDA that metal particles were found in some of their GENTLEASE infant formula. About 41,464 cans of the product were distributed beginning in December 2005 through retail stores nationwide. No illnesses had been reported at the time of the recall, but officials were concerned the particles could damage a baby's respiratory system and throat, causing coughing, difficulty swallowing, or difficulty breathing.
In other instances, a company recalls a product after FDA raises concerns. This action could occur after the agency inspects a manufacturing facility or evaluates reports of health problems. For example, the Cold Stone Creamery removed all of its "cake batter" ice cream products from store shelves in July 2005 after the FDA told the company that several cases of Salmonella Typhimurium infection occurred among people who had eaten the cake batter products. This type of salmonella can cause abdominal pain, high fever, nausea, and vomiting in healthy people and can cause serious and sometimes fatal infections in small children, older people, and in those with weakened immune systems.
In rare cases, FDA requests that a manufacturer recall a product. An example of this type of recall occurred in February 2006, when FDA asked Cytosol Laboratories Inc. to recall all brands and sizes of its Balanced Salt Solution (BSS), which health professionals use to irrigate patients' eyes, ears, nose, or throat during cataract surgery and other procedures. FDA requested the recall because product lots were found to have elevated levels of endotoxins, substances found in certain bacteria that can cause fever, shock, and changes in blood pressure and in other circulatory functions.
FDA received reports of a serious eye injury called Toxic Anterior Segment Syndrome (TASS), as well as complaints relating to injuries in more than 300 people who were given BSS. An estimated 1 million units of BSS products manufactured by Cytosol were distributed between December 2003 and December 2005. In response to FDA's request, Cytosol voluntarily recalled several of its products.
Whatever the reason for a voluntary recall, FDA's role is to oversee the company's recall strategy. In 2003, the agency released guidance for industry on managing all aspects of a recall. The guidance assists a company in recall strategy development, and encourages them to provide the information FDA needs to evaluate and classify the recall.
Evaluating the Health Hazard
For any product regulated by FDA, a recall is handled by the appropriate FDA product center. A recall of a blood product, for example, would be addressed by the FDA's Center for Biologics Evaluation and Research. A recall of a drug for dogs would fall with the FDA's Center for Veterinary Medicine.
Did You Know?
In 2005, more than 5,300 products regulated by FDA were recalled. Of these products, 466 were Class I, 3,781 were Class II, and 1,091 were Class III. Most Class I recalls were in
the food category. |
Once a recalled product is referred, the center assesses the risk (health hazard) it poses to the public. Experts evaluate factors such as whether any diseases and injuries have already occurred; what risks apply to various segments of the population, including children, pets, and surgical patients; the seriousness of the health hazard; and the volume of product involved and the distribution.
Based on all of this information, the experts assign the recall a classification to indicate the degree of the health hazard, with Class I being the most serious.
Here's a look at the classes of recalls:
Class I recalls involve a situation in which there is reasonable probability that use of or exposure to the product will cause serious health problems or death.
Class II recalls mean that use of or exposure to the product may cause temporary or medically reversible health problems or that the probability of serious health problems is remote.
Class III recalls are the least serious and indicate exposure to the product is not likely to cause health problems.
Notifying the Public
An evaluation of the health hazard helps companies determine the strategy for communicating risks associated with a recalled product. As part of reviewing a company's proposed recall strategy, FDA looks at the depth of the recall, the extent of public warnings needed, and whether the recall is being extended to wholesale, hospital, pharmacy, retail, patient, or consumers.
If companies sell directly to consumers and can identify them easily, a letter to the consumer could work. There are some situations where only consumers know whether or not they bought a product and the approach must be much broader in order to notify as many people as possible.
Class I recalls almost always warrant a release to the media. When a recalled product has been widely distributed, the media is a very effective way to reach large numbers of consumers.
Class II and III recalls aren't announced in the media, but they go into the FDA's weekly Enforcement Report, posted at http://www.fda.gov/opacom/Enforce.html on the agency's Web site. This document lists each recall according to classification, with the specific action taken by the recalling firm.
Companies must report to FDA whether the recall will be posted on the company's Web site, what customers are being instructed to do, and how products should be returned, if applicable. Key information should be communicated clearly to consumers, including
- the name of the product
- the lot numbers
- serial numbers, or other identifying information
- the reason for the recall
Consumers need to take any recall communication seriously and determine whether they are affected. In some cases, it may be that only a small portion of the product is actually affected. But to be on the safe side and to operate within current good manufacturing practice regulations, the entire product line, lot, or model may be recalled.
An example of a case in which consumers were instructed to return recalled product was when RC2 Brands recalled certain teething rings in January 2006. The liquid in the rings was contaminated with bacteria that could cause serious illness if swallowed by babies, if it enters the lungs, or if it is absorbed through a cut in the mouth. The risk of illness is especially high in infants whose immune systems are compromised by malnutrition, by blood problems, or from cancer therapy. As of February 2006, the firm had received 105 complaints of fluid leakage, 14 reports of sharp edges that resulted in nine incidents of cuts, and two reports of babies biting through rings.
For this recall, the firm asked consumers to place the teethers in a plastic bag and return them to the company, but consumers should be aware that some recalls don't involve returning products. Sometimes, the recall doesn't involve a removal, but rather a correction. This solution is a way to address a problem with the product where it's sold. For example, a medical device in a hospital might need a new label or some kind of reprogramming.
A recall also may be issued when a company has released new warnings and instructions for a device. In June 2005, Vail Products Inc. recalled about 5,000 enclosed or canopied bed systems because patients could become entrapped or suffocate.
At the time of the recall, the FDA was aware of 30 entrapments, of eight of which resulted in death. The company did not retrieve or replace the beds; they couldn't be returned to the company. But the company provided new instruction manuals and warning labels, and stopped manufacturing, selling, and distributing the bed systems.
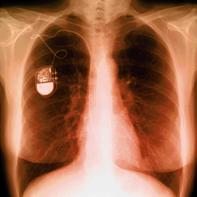 With an implanted device, a recall may mean that doctors and patients should discuss the risks of removing the device compared to the risk of leaving it in place. That’s what happened when Guidant Corporation announced a voluntary Class I recall in July 2005 of certain pacemakers that were manufactured between November 25, 1997, and October 26, 2000. With an implanted device, a recall may mean that doctors and patients should discuss the risks of removing the device compared to the risk of leaving it in place. That’s what happened when Guidant Corporation announced a voluntary Class I recall in July 2005 of certain pacemakers that were manufactured between November 25, 1997, and October 26, 2000.
A seal within the devices can leak, allowing moisture to affect the electronic circuits. This defect can prevent the pacemaker from providing pacing or can cause a rapid heart rate. The problems could occur without warning and lead to loss of consciousness, heart failure, or death. As of July 2005, Guidant had received reports that 69 pacemakers that may have failed because of the leakage.
In a letter to physicians, Guidant gave physicians recommendations about how to identify a leak-related malfunction and other advice for minimizing the risk of pacemaker failure.
Preventing Future Problems
Companies should work with the FDA to identify root causes for a recall and to prevent the problem from recurring. FDA's role is to oversee the recall and verify that the firm's steps reflect the right conclusions about the problem and how it should be corrected.
In December 2005, Diamond Pet Food traced the cause of injuries and deaths of dozens of dogs to a potent toxin called aflatoxin, which was found in some of the pet food manufactured at the company's facility in Gaston, South Carolina. The aflatoxin came from a fungus found on corn that was used to make the food. Experts say that severe drought followed by high moisture contributes to the growth of this fungus.
Animals that consume aflatoxin may experience severe liver damage. Signs of illness in pets include sluggishness, lethargy and reluctance to eat, a yellowish tint to the eyes and gums, and severe or bloody diarrhea.
From the first week of the recall, Diamond Pet Food worked with FDA to get the word out to potentially affected households and improve their food safety procedures and protocols.
Diamond Pet Food has taken several actions to prevent oversights from happening again. The company implemented sampling and testing protocols for corn that require two signatures throughout the chain of custody--that of the employee and the immediate supervisor. They also conduct 12 quality tests on each load of corn rather than the four required by the USDA.
Diamond also added aflatoxin testing to the finished product and is working with the USDA to source corn from areas that are less likely to have aflatoxin-tainted supplies.
Evaluating Effectiveness
Companies carry out the recall, and FDA acts as a monitor. FDA encourages companies to make sure that recall notification letters reached the target audience, that the letter was read, and that instructions were understood.
Classification of the recall helps FDA determine the number of audits needed to check on a recall. An audit check, whether done by personal visit, phone call, letter, or a combination, is made so that FDA can verify who has been notified about the recall. Check levels range from A to E. A "Level A" check means that 100 percent of those targeted were contacted about the recall. A "Level E" check means that no effectiveness checks were needed.
FDA requests recalling firms submit periodic status reports indicating how many people are responding to the recall. The recall is considered complete after all of the company's corrective actions are reviewed and deemed appropriate. FDA evaluates whether all reasonable efforts have been made to remove or correct the product, and whether all outstanding product is recovered or destroyed.
When the Recall Is Not Voluntary
Though most recalls are voluntary, there are times when FDA mandates a recall. FDA can "order" a recall in some cases involving infant formulas, biological products, and devices that present a serious hazard to health. In 2002, FDA ordered CyroLife Inc. to recall and prevent further use of human tissue that was processed from October 2001 to August 2002.
During an inspection in 2002, FDA found significant violations of FDA regulations and issued a Warning Letter. FDA issued the recall order after determining that CryoLife had failed to take adequate measures to address possible infectious disease contamination of tissue. Tissue from a donor processed by CryoLife was associated with the November 7, 2001 death of a patient who received a soft tissue implant during reconstructive knee surgery.
Did You Know?
When companies are unwilling to comply with the law, FDA takes decisive action to protect consumers. This sends a strong signal that the agency doesn't tolerate harmful products in the marketplace. |
If FDA experts determine a recall wouldn't be effective or if a recall proves ineffective, or if the violation is continuing, then the agency can take legal action such as injunctions and seizures. An injunction is a civil action taken to stop production or distribution of a product that violates the law. A seizure involves removing a product from the market by requesting a court to direct a U.S. Marshal to take possession of the goods, usually from the manufacturer’s warehouse.
FDA conducted a seizure in May 2002 of New Choice Food mini-gel candies at the company's facility in Irwindale, California. In 2001, FDA had issued warnings against consuming mini-cup gel candies that contain the ingredient "konjac," also known as conjac, konnyaku, yam flour, or glucomannan. FDA and the Consumer Product Safety Commission considered this product a choking hazard, especially to children and older people. The candy, which was sold under various brand names and distributed by different companies, doesn't readily dissolve in the mouth.
At the time of the seizure, six deaths had been reported from choking in the U.S., and deaths were also reported in other countries. FDA issued an import alert in 2001 to keep the candy out of the U.S. Other firms voluntarily recalled the gel candies, but New Choice Food did not.
The recall process is an important tool used by FDA to protect public health. Most recalls are done voluntarily by the manufacturer and monitored by the FDA; a small number are the result of FDA legal action. The result is safer products for consumers.
For More Information About FDA Recalls
Sign Up for Recall Alerts
News releases about recalls of products regulated by the FDA can be delivered directly to an e-mail account.
Visit FDA's free e-mail lists page at http://www.fda.gov/emaillist.html and click on "FDA Recalls."
Source: FDA Consumer
Back to Table of Contents
Calendar
of National Health Events
September
National Food Safety Education Month
International Food Safety Council
National Restaurant Association Education Foundation
175 West Jackson, Suite 1500
Chicago, IL 60604
(312) 715-1010 x712
Bsirt@foodtrain.org
www.nraef.org/index.asp
Materials available
Contact: LeAnn Chuboffe
October
Halloween Safety Month
Prevent Blindness America
500 East Remington Road
Schaumburg, IL 60173
(800) 331–2020
info@preventblindness.org
www.preventblindness.org
Materials available
Contact: PBA Consumer and Patient Hotline
November
American Diabetes Month
American Diabetes Association
1701 North Beauregard Street
Alexandria, VA 22311
(800) DIABETES (342-2383)
askada@diabetes.org
www.diabetes.org
Materials available
Contact: Local Affiliates
Other health events that may be of interest to teens are listed on our website at
http://www.fda.gov/cdrh/fdaandyou/calendar.html
Back to Table of Contents
Crossword
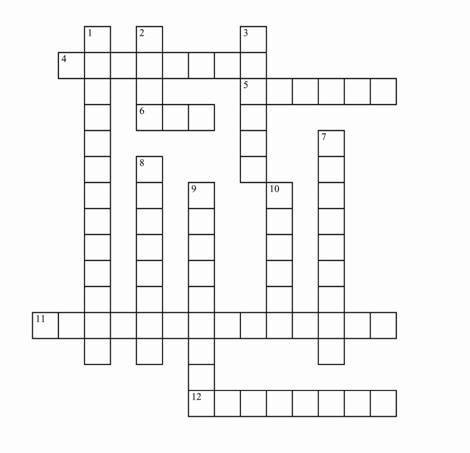
Across
4 Substances that change the way your body works
5 A protein found in milk
6 Disease that can cause genital warts
11 The LUMA Cervical Imaging System helps doctors detect this disease
12 One of the eight allergenic foods
Down
1 Can help predict disease in families
2 New educational program on the safe use of OTC medicines
3 Remove or correct products that violate laws enforced by FDA
7 Products intended to alter the appearance
8 Cervical cancer vaccine containing no live virus
9 Lists a medicine’s active ingredients
10 New food labeling law
Back to Table of Contents
| About FDA & You
FDA & You is an FDA publication to inform and encourage health educators and students to learn about the latest FDA medical device and health news.
The publication's contents may be freely reproduced. Comments should be sent to the Editor.
Editor: Alicia Witters
Editor: Edie Seligson
Researcher: Carol Clayton
Lesson Plans: Tamara Wirt
Email: FDAandyou@cdrh.fda.gov
Read us online at:
http://www.fda.gov/cdrh/fdaandyou
Department of Health and Human Services, Food and Drug Administration
Center for Devices and Radiological Health
Rockville, MD 20850
Special thanks to: CBER, CDER, CDRH, CFSAN, and FDA Consumer for contributing to this issue. |
Crossword Solutions
Across: 4 Medicine, 5 Casein, 6 HPV, 11 Cervical Cancer, 12 Soybeans
Down: 1 Health History, 2 MIMH, 3 Recall, 7 Cosmetics, 8 Gardasil, 9 Drug Facts, 10 FALCPA
|










![]()