|
|||||
|
|
|||||
|
|
|||||
Table of Contents |
NARMS Retail Meat Annual Report 2004 - Introduction
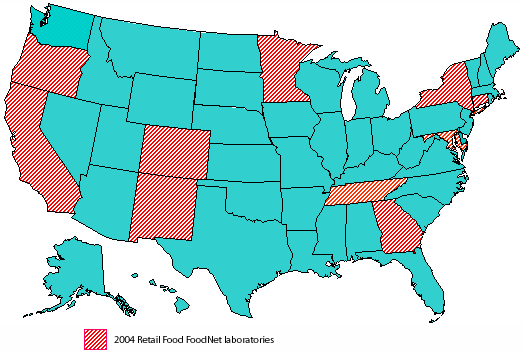
Background: Food animal products destined for human consumption are known to harbor enteric bacteria, including zoonotic foodborne pathogens. Antimicrobial resistance (AR) among these organisms may be associated with the use of antimicrobial agents in food animals. Retail meats represent a point of exposure close to the consumer and, when combined with data from slaughter plants and on-farm studies, provides insight into the prevalence of AR in foodborne pathogens originating from food animals. To gain a better understanding of AR among enteric bacteria in the food supply, the NARMS monitors antimicrobial susceptibility/resistance phenotypes in bacteria isolated from retail meats. The primary purpose of the NARMS retail meat surveillance program is to monitor the prevalence of antimicrobial resistance among foodborne pathogenic and commensal organisms, in particular, Salmonella, Campylobacter, Enterococcus and E. coli The results generated by the NARMS retail meat program will establish a reference point for analyzing trends of antimicrobial resistance among these foodborne bacteria. NARMS retail meat surveillance is an ongoing collaboration between the U.S. Food and Drug Administration (Center for Veterinary Medicine), the Centers for Disease Control and Prevention, and in 2004, all 10 of the current FoodNet laboratories: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Retail meats are collected at these FoodNet sites and cultured for the presence of the selected organisms. Bacterial isolates are sent to FDA/CVM for confirmation of species, antimicrobial susceptibility testing, and genetic analysis.
Retail meat sampling: For calendar year 2004, retail meat sampling started in January among the 10 participating FoodNet laboratories. In each of the FoodNet sites monthly sampling, an attempt was made to go to as many different stores as possible. The object was to purchase as many different brands of fresh (not frozen) meat and poultry as possible. A total of 40 food samples were purchased per month comprised of 10 samples each of chicken breast, ground turkey, ground beef, and pork chops. For each meat and poultry sample, the FoodNet sites recorded the store name, brand name, lot number (if available), sell-by date, purchase date and lab processing date on log sheets (A-9). Additional information with regard to whether or not the meat or poultry was ground or cut in-store was also collected, if possible. Samples were kept cold during transport from the grocery store(s) to the laboratory. Microbiological analysis: In the laboratory, samples were refrigerated at 4°C and were processed no later than 96 hours after purchase. After microbiological examination, the sites recorded on the log sheets whether or not the meat and poultry samples were presumptively positive for Salmonella, Campylobacter, E. coli, and Enterococcus. Each laboratory used essentially the same procedure for sample collection. Retail meat and poultry packages were kept intact until they were aseptically opened in the laboratory at the start of examination. For chicken and pork samples, one piece of meat was examined. For ground beef and ground turkey samples, 25 g of ground product was analyzed. The analytical portions from each sample were placed in separate sterile plastic bags, 250 mL of buffered peptone water was added to each bag, and the bags were vigorously shaken. Fifty mL of the rinsate from each sample was transferred to separate sterile containers for isolation and identification of Salmonella, Campylobacter, E. coli, or Enterococcus using standard microbiological procedures. Once isolated and identified, bacterial isolates were sent to FDA’s CVM Office of Research for further characterization including species confirmation, antimicrobial susceptibility testing and PFGE analysis (Salmonella and Campylobacter only). Changes in 2004 Several notable updates in the NARMS Retail Meat program occurred in 2004. A total of 4699 meats samples were collected, up from 3533 in 2003. This was due to the addition of FoodNet laboratories in Colorado and New Mexico, increasing the number of test sites from 8 to 10. The interpretive criteria used for Campylobacter antimicrobials is shown in Table 1. Based on the upcoming CLSI M45-P document (Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently-Isolated or Fastidious Bacteria; Proposed Guideline, CLSI June 2006), the Erythromycin resistance breakpoint was changed from 8 µg/ml to 32 µg/ml. Based on the MIC distribution published in this report, along with other Campylobacter data generated using broth microdilution testing, several other breakpoints have been modified from those used in previous NARMS reports. For resistance breakpoints, these revised values include: Azithromycin (changed from 2 µg/mL to 8 µg/ml); Clindamycin (changed from 4 µg/mL to 8µg/ml); Gentamicin (changed from 16 µg/mL to 8 µg/ml); and Nalidixic acid (changed from 32 µg/mL to 64 µg/mL). NARMS retail meat working group, 2004 U.S. Food and Drug Administration Jason Abbott Centers for Disease Control and Prevention Dr. Fred AnguloCalifornia Richard Alexander Connecticut Robert Howard Colorado Joe GossackGeorgia James Benson Maryland Karen Cuenco Minnesota Craig Braymen New Mexico Lisa Butler New York Gina Conenello Oregon Debbie Bergquist Tennessee Samir HannaAcknowledgements Much thanks to Deborah Brooks and Michelle Talley for providing outstanding web support to the NARMS program.
Web Page Updated by hd - August 14, 2006, 9:40 AM ET
|