Beth Baseler, MS
Director, CMRP, SAIC-Frederick, Inc.
Phone: 301-846-5413, Fax: 301-846-6188
E-mail: bbaseler@mail.nih.gov
Director, CMRP, SAIC-Frederick, Inc.
Phone: 301-846-5413, Fax: 301-846-6188
E-mail: bbaseler@mail.nih.gov
MISSION STATEMENT
The primary mission of the Clinical Monitoring Research Program (CMRP) is to extend an important facet of clinical research support for the NIH by providing comprehensive dedicated clinical research support to major programs within the NCI and NIAID.
OVERVIEW
The Clinical Monitoring Research Program (CMRP) provides comprehensive dedicated clinical research support to major programs within the National Cancer Institute (NCI), including the Center for Clinical Research (CCR), Division of Cancer Control and Population Sciences (DCCPS), the Division of Cancer Treatment and Diagnosis (DCTD), and the National Institute of Allergy and Infectious Diseases. CMRP staff has continued to provide high-quality programmatic and clinical trials management support to an extensive variety of high-profile NCI and NIAID initiatives.
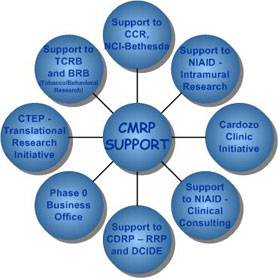
The CMRP includes:
1) Regulatory Compliance and Human Subjects Protection Program (RCHSPP), providing support to NIAID
2) Support to the NIAID-Mali HIV Research Initiative
3) Support to the Phidisa Project, a joint effort between the South African Military Health Service of the South African National Defense Force, the U.S. Department of Defense (DoD), and the National Institutes of Health (NIH)
4) Clinical Consulting and Services Group
5) Support to the India/Mali Initiative
6) Support to the Nigeria Project
7) Support to Biostatistics
8) Support to the BioDefense Clinical Research Branch
9) Support Uganda Initiatives
10) Nursing and clinical/protocol monitoring support to the CCR
11) Support to the Cardozo Clinic Initiative
12) The Translational Research Initiative providing support to the Clinical Therapy Evaluation Program (CTEP)
13) Tobacco Control Research Branch (TCRB)
14) Support to the Development of Clinical Imaging Drugs and Enhancers (DCIDE) program
15) Cancer Disparities Research Program
16) Support to the Health Communication and Informatics Research Branch
17) Support to the CCR/DCTD Phase 0 Initiative
Major efforts continue to include the management of a clinical trials management and regulatory compliance program to monitor an extensive variety of clinical trials being conducted by the Intramural Research Program within NIAID; major programmatic support to the NIAID-Mali HIV Research Initiative located in Bamako, Mali; major programmatic support to a variety of emerging infectious diseases initiatives; and management of subcontracts to support correlative studies sponsored by the Translational Research Initiative sponsored by NCI's Cancer Therapy Evaluation Program. The group also continues to play major role in providing clinical research nursing and protocol support for the NCI's CCR in Bethesda. A recent addition to our program includes grant management support to the NCI's Cancer Disparities Research Partnership within the Radiation Oncology Research Program, DCTD, and information technology management and regulatory/clinical monitoring support for DCIDE, Cancer Imaging Program, DCTD.
The CMRP includes:
1) Regulatory Compliance and Human Subjects Protection Program (RCHSPP), providing support to NIAID
2) Support to the NIAID-Mali HIV Research Initiative
3) Support to the Phidisa Project, a joint effort between the South African Military Health Service of the South African National Defense Force, the U.S. Department of Defense (DoD), and the National Institutes of Health (NIH)
4) Clinical Consulting and Services Group
5) Support to the India/Mali Initiative
6) Support to the Nigeria Project
7) Support to Biostatistics
8) Support to the BioDefense Clinical Research Branch
9) Support Uganda Initiatives
10) Nursing and clinical/protocol monitoring support to the CCR
11) Support to the Cardozo Clinic Initiative
12) The Translational Research Initiative providing support to the Clinical Therapy Evaluation Program (CTEP)
13) Tobacco Control Research Branch (TCRB)
14) Support to the Development of Clinical Imaging Drugs and Enhancers (DCIDE) program
15) Cancer Disparities Research Program
16) Support to the Health Communication and Informatics Research Branch
17) Support to the CCR/DCTD Phase 0 Initiative
Major efforts continue to include the management of a clinical trials management and regulatory compliance program to monitor an extensive variety of clinical trials being conducted by the Intramural Research Program within NIAID; major programmatic support to the NIAID-Mali HIV Research Initiative located in Bamako, Mali; major programmatic support to a variety of emerging infectious diseases initiatives; and management of subcontracts to support correlative studies sponsored by the Translational Research Initiative sponsored by NCI's Cancer Therapy Evaluation Program. The group also continues to play major role in providing clinical research nursing and protocol support for the NCI's CCR in Bethesda. A recent addition to our program includes grant management support to the NCI's Cancer Disparities Research Partnership within the Radiation Oncology Research Program, DCTD, and information technology management and regulatory/clinical monitoring support for DCIDE, Cancer Imaging Program, DCTD.
