You are here:
Research
- Office Overview
- Biodata Mining and Discovery Section
- Career Development Section
- Engineering and Instrumentation Unit
- Flow Cytometry Section
- Laboratory Animal Care and Use Section
- Light Imaging Section
- Macromolecular Biophysics Section
- Translational Immunology Section

Flow Cytometry Section
Jim Simone
Section Leader, Flow Cytometry Section
Office of Science and Technology (OST)
Phone: (301) 594-3531
Fax: (301) 402-2209
E-mail: simonej@mail.nih.gov
Research Overview
The primary goal of the Flow Cytometry Facility is to provide the services of state-of-the-art multi-parameter cellular analysis and cell sorting for researchers and investigators of the NIAMS IRP. The Facility currently includes two FACSCaliburs (2-laser 4-color analyzer), a DakoCytomation CyAn (3-laser 9-color analyzer) and a DakoCytomation high-speed sorter, MoFlo, which can detect up to 10 colors with digital pulse processing. The Facility maintains and updates the equipment as needed, provides training for investigators, schedules sorting, and collaborates for creative solutions to scientific experiment. Particular interests include multi-parameter analysis, fluorescence resonance energy transfer, single cell sorting, and cytometric analysis of intracellular signaling events.
Flow cytometry technology and its research applications are the primary focus of work by Dr. Liusheng He and his colleagues. This vanguard technology plays an increasingly important role in studies of the fundamental molecular processes of immune responses to exogenous antigens as well as self-antigens, and also in tumor development and tumor suppression. Dr. He has participated in the development and application of multiple-laser Fluorescence-Activated Cell Sorter (FACS) systems for biological investigations. His current work seeks to advance the capability of the FACS technology to permit simultaneous identification of up to ten fluorochrome tagged cellular markers.
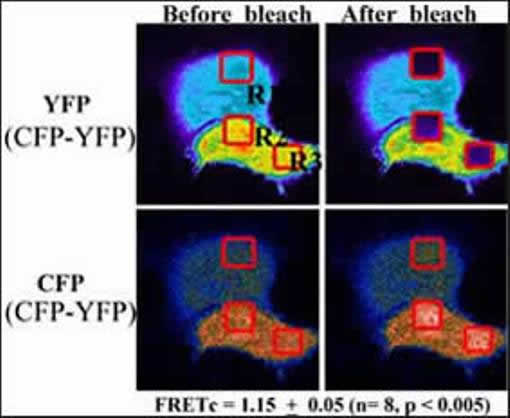
In the Flow Cytometry Facility, FACS technology is being utilized to study intracellular signaling pathways involved in defining the pathways of apoptosis or of survival signals. This process involves the formation of protein complexes mediating signal transduction from cell surface receptors to the nuclear genetic machinery. Over the last several years, Dr. He employed multiple-laser FACS to establish and develop a cutting-edge FRET (fluorescent resonance energy transfer) technique in the Facility, including the developments of methods of detecting FRET by a single laser and for the first time of how to calculate FRET efficiency on flow cytometric data. More recently, Dr. He has described a novel methodology to monitor protein-protein interactions between three proteins, unlike most FRET works being processed between two proteins. Currently, Dr.He and his colleagues are applying this novel technique for more biological investigations, such as CD154-CD40 signaling through TRAF molecules and monitoring two distinct caspase activities in living cells.

Selected Publications
He L, Wu X, Simone J, Hewgill D, Lipsky PE. Determination of tumor necrosis factor receptor-associated factor trimerization in living cells by CFP->YFP->mRFP FRET detected by flow cytometry. Nucleic Acids Res. 2005; 33(6):e61.
![]()
He L, Wu X, Meylan F, Olson DP, Simone J, Hewgill D, Siegel R, Lipsky PE. Monitoring caspase activity in living cells using fluorescent proteins and flow cytometry. Am J Pathol. 2004; 164(6): 1901-13.
![]()
Karpova TS, Baumann CT, He L, Wu X, Grammer A, Lipsky P, Hager GL, McNally JG. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc. 2003; 209(Pt 1): 56-70.
![]()
He L, Olson DP, Wu X, Karpova TS, McNally JG, Lipsky PE. A flow cytometric method to detect protein-protein interaction in living cells by directly visualizing donor fluorophore quenching during CFP->YFP fluorescence resonance energy transfer (FRET). Cytometry. 2003; 55A(2): 71-85.
![]()
He L, Bradrick TD, Karpova TS, Wu X, Fox MH, Fischer R , McNally JG, Knutson JR, Grammer AC, Lipsky PE. Flow cytometric measurement of Fluorescence Resonance Energy Transfer from cyan fluorescent protein to yellow fluorescent protein using single laser excitation at 458 nm. Cytometry. 2003; 53A(1): 39-54.
![]()
Updated September 17, 2007



