| January 13, 2009

[NIST Tech Beat Search] [Credits] [NIST Tech Beat Archives] [Media Contacts] [Subscription Information]

New NIST Method Accelerates Stability Testing of Soy-Based Biofuel
 |
NIST Chemist Tom Bruno demonstrates sampling of biodiesel fuel for injection into a gas chromatograph-mass spectrometer, an instrument that separates and identifies the components of a mixture.
Credit: Ost, NIST
View hi-resolution image |
The National Institute of Standards and Technology (NIST) has developed a method to accelerate stability testing of biodiesel fuel made from soybeans and also identified additives that enhance stability at high temperatures. The results, described in a new paper,* could help overcome a key barrier to practical use of biofuels.
Both oxidation and heating can cause biodiesel to break down, adversely affecting performance. These two effects usually are analyzed separately, but NIST chemists developed a method to approximate both effects at the same time while also analyzing fluid composition. NIST’s “advanced distillation curve” method could accelerate and simplify testing of biodiesels, according to lead author Tom Bruno. NIST researchers used the new method to demonstrate the effectiveness of three additives in reducing oxidation of biodiesel at high temperatures, as would occur in aviation fuels.
Biodiesel—which can be prepared from vegetable oil, animal fats, used cooking oil, or microalgae—is a potential replacement or extender for petroleum-based diesel fuel. Biodiesel offers several advantages, including renewability, the potential for domestic production, biodegradability, and decreased emissions of carbon monoxide and particulate matter. Biodiesel also has several serious disadvantages, including increased nitrogen oxide emissions and chemical instability, especially at higher temperatures.
Antioxidants often are added to vegetable oils to retard oxidation during storage. The NIST work may be the first to enhance stability of biofuel at high temperatures, Bruno said. The study focused on three compounds, THQ, t-decalin and tetralin,** that help neutralize highly reactive “free radicals” formed at temperatures above 300 degrees C. Test results showed that all three compounds stabilized biodiesel. As expected from studies of aviation fuels, THQ and t-decalin perform similarly and outperform tetralin. For solutions containing 1 percent additive, THQ performed best overall.
A distillation curve charts the percentage of a mixture that evaporates as a sample is slowly heated. Because the different components of a complex mixture typically have different boiling points, a distillation curve gives a good measure of the relative amount of each component. NIST chemists enhanced the traditional technique by improving precision and control of temperature measurements and adding the capability to analyze the chemical composition of each boiling fraction.
To adapt the method for unstable fluids such as biodiesels, the authors made repeated distillation curves of samples and quantified the variation in parameters such as temperature for each distillate fraction across the different runs of the experiment. These data were averaged over the entire distillation curve to identify the range of variations that might occur. This range was extended to theoretically model the potential oxidative and thermal decomposition of the samples.
Two authors of the new paper participated in NIST’s Summer Undergraduate Research Fellowship (SURF) program. (Learn more about the SURF program at NIST in Gaithersburg, Md. and Boulder, Colo.)
* T.J. Bruno, A. Wolk and A. Naydich. Stabilization of biodiesel fuel at elevated temperature with hydrogen donors: Evaluation with the advanced distillation curve method. Energy & Fuels. Articles ASAP (Web), January 2, 2009. DOI: 10.1021/ef800740d.
** THQ: 1,2,3,4-tetrahydroquinoline; t-decalin: transdecahydronaphthalene; tetralin: 1,2,3,4-tetrahydronaphthalene.
Edited Jan. 14, 2008 to expand the SURF links.
Media Contact: Laura Ost, laura.ost@nist.gov, (303) 497-4880 

New Tool Gives Researchers a Glimpse of Biomolecules in Motion
 |
NIST researcher Ted Heilweil, National Research Council postdoctoral fellow Catherine Cooksey (pictured), and NIST Summer Undergraduate Research Fellow Ben Greer from Carnegie Mellon University have demonstrated the feasibility of a new technique for studying biomolecules using terahertz radiation. Because terahertz waves are almost completely absorbed by water, the team was able to reduce the amount of water to the bare minimum while still providing a realistic sample environment by using hollow, nanosized droplets called micelles as tiny test tubes.
Credit: NIST
View hi-resolution image |
The ability of biomolecules to flex and bend is important for the performance of many functions within living cells. However, researchers interested in how biomolecules such as amino acids and proteins function have long had to make inferences from a series of X-ray-like “still pictures” of pure crystalline samples. Now, using a new technique based on terahertz (THz) spectroscopy, scientists at the National Institute of Standards and Technology (NIST) have recently taken the first step toward revealing the hidden machinations of biomolecules in water.*
With wavelengths that range from 1 millimeter to 25 micrometers, terahertz radiation falls between the infrared and microwave spectral regions. Researchers can determine how molecules are moving by passing terahertz radiation through a sample and measuring which wavelengths are absorbed. Unfortunately, room temperature water, the medium in which biological molecules typically are studied, absorbs nearly all of the terahertz radiation, limiting the utility of terahertz spectroscopy for probing biomolecular function.
To avoid the water problem, the NIST team needed to find a way to provide a simple but realistic environment for the biomolecules that contained the least amount of water possible. NIST researcher Ted Heilweil, National Research Council postdoctoral fellow Catherine Cooksey and NIST Summer Undergraduate Research Fellow Ben Greer from Carnegie Mellon University found their solution in the form of nanoscale droplets made of soap-like molecules called micelles.
Using the micelles as tiny test tubes, the team filled the hollow molecules with a small sample of water and the amino acid L-proline, a protein building block. Measurements validated their hypothesis that the micelles would provide an aqueous environment that allows the amino acid to flex and bend while limiting the absorption of the terahertz radiation by water. The terahertz measurements on this simple biomolecule compared well with expectations from other studies, further validating the technique.
According to Heilweil, this study is an important first step toward using terahertz radiation for studying biomolecules. More ambitious measurements on larger molecules such as small peptides, proteins, and DNA fragments will be more challenging, but he says it may be possible in the near future.
“If we can get larger molecules in [the micelles], we can get a much better idea of how living molecules function,” Heilweil said. “This will let us see the basic, most fundamental building blocks of life as they move, which is very exciting.”
* C. Cooksey, B. J. Greer and E. J. Heilweil. Terahertz spectroscopy of l-proline in reverse aqueous micelles. Chemical Physics Letters. Available online Nov. 21, 2008.
Media Contact: Mark Esser, mark.esser@nist.gov, (301) 975-8735 

‘Two-Faced’ Bioacids Put a New Face on Carbon Nanotube Self-Assembly
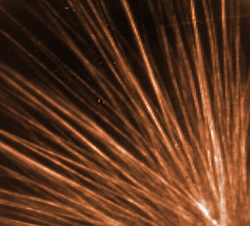 |
Single wall carbon nanotubes enclosed in bile acid shells self assembled into a sheaf of long ordered fibrils each composed of several nanotube rods. Treating the microscope slide with a hydrophobic compound causes the fibrils to cluster like this at specific sites, probably at defects in the hydrophobic surface. Image, 70 micrometers wide, was taken using near-infrared fluorescent microscopy. (Color added for clarity.)
Credit: NIST
View hi-resolution image |
Nanotubes, the tiny honeycomb cylinders of carbon atoms only a few nanometers wide, are perhaps the signature material of modern engineering research, but actually trying to organize the atomic scale rods is notoriously like herding cats. A new study* from the National Institute of Standards and Technology (NIST) and Rice University, however, offers an inexpensive process that gets nanotubes to obediently line themselves up—that is, self-assemble—in neat rows, more like ducks.
A broad range of emerging electronic and materials technologies take advantage of the unique physical, optical and electrical properties of carbon nanotubes, but most of them—nanoscale conductors or “nanowires,” for instance—are predicated on the ability to efficiently line the nanotubes up in some organized arrangement. Unfortunately, just mixed in a solvent, the nanotubes will clump together in a black goo. They can be coated with another molecule to prevent clumping—DNA is sometimes used—but spread the mixture out and dry it and you get a random, tangled mat of nanotubes. There have been a variety of mechanical approaches to orienting carbon nanotubes on a surface (see, for example, “NIST’s Stretching Exercises Shed New Light on Nanotubes,” Tech Beat, Apr. 12, 2007), but a more elegant and attractive solution would be to get them to do it themselves—self assembly.
NIST researchers studying better ways to sort and purify carbon nanotubes to prepare standard samples of the material were using a bile acid** to coat the nanotubes to prevent clumping. “Bile acids,” says NIST research chemist Erik Hobbie, “are biological surfactants, and like most surfactants they have a part that likes water and a part that doesn’t. This is a slightly complex surfactant because instead of having a head and a tail, the usual geometry, it has two faces, one that likes water and one that doesn’t.” Mixed in water, such hydrophobic/hydrophilic molecules normally want to group together in hollow spheres with their hydrophobic “tails” sheltered on the inside, Hobbie explains, but the two-faced geometry of this bile acid makes it form hollow rod shapes instead. Conveniently, the hollow rods can house the rod-shaped nanotubes.
As it turns out, there’s a bonus. Over the course of about a day, the bile acid shells cause the nanotubes to begin lining up, end to end, in long strands, and then the strands begin to join together in twisted filaments, like a length of twisted copper wire. The discovery is a long way from a perfect solution for ordering nanotubes, Hobbie cautions, and a lot of development remains to be done. For one thing, ideally, the bile acid shells would be removed after the nanotubes are in their ordered positions, but this has proven difficult. And the surfactant is toxic to living cells, which precludes most biomedical applications unless it is removed. On the other hand, he says, it already is an easy and extremely inexpensive technique for researchers interested in studying, for example, optical properties of carbon nanotubes. “It gives a recipe for how to create ordered, aligned arrangements of individual carbon nanotubes. You don’t need to use any external magnetic or electrical fields, and you don’t need to dry the tubes out in a polymer and heat it up and stretch it. You can get fairly significant regions of very nice alignment just spontaneously through this self assembly.”
(For more on the purifying of carbon nanotubes, see “Spin Control: New Technique Sorts Nanotubes by Length,” Tech Beat, May 13,2008.)
* E.K. Hobbie, J.A. Fagan, M.L. Becker, S.D. Hudson, N. Fakhri and M. Pasquali. Self-assembly of ordered nanowires in biological suspensions of single-wall carbon nanotubes. ACS Nano, published online Dec. 16, 2008.
** Sodium deoxycholate.
Media Contact: Michael Baum, michael.baum@nist.gov, (301) 975-2763 

Super Sensitive Gas Detector Goes Down the Nanotubes
 |
NIST researchers have developed a new technique to form nanotubes for use in gas sensing applications. One hundred to 1,000 times more sensitive than comparable sensors, their device could be used to study biological cell stress and cell communication.
Credit: Artzi-Gerlitz, NIST
View hi-resolution image
View schematic of the nanotube sensor |
When cells are under stress, they blow off steam by releasing minute amounts of nitrogen oxides and other toxic gases. In a recent paper,* researchers at the National Institute of Standards and Technology (NIST) described a new method for creating gas detectors so sensitive that some day they may be able to register these tiny emissions from a single cell, providing a new way to determine if drugs or nanoparticles harm cells or to study how cells communicate with one another. Based on metal oxide nanotubes, the new sensors are a hundred to 1,000 times more sensitive than current devices based on thin films and are able to act as multiple sensors simultaneously.
Gas sensors often operate by detecting the subtle changes that deposited gas molecules make in the way electricity moves through a surface layer. Thus, the more surface available, the more sensitive the sensor will be. Scientists are interested in developing gas sensors based on nanotubes because, having walls that are only a few nanometers thick, they are almost all surface.
Although nanotubes have proven to be well suited for gas sensing applications, fabricating the devices themselves is a difficult, imprecise and time-consuming process, according to Kurt Benkstein, an author of the paper. Older methods include randomly scattering free nanotubes on a surface with preformed electrical contacts (the hope being that a least a few of the nanotubes would tumble into place) or laying contacts over the top of the nanotubes after they had been dispersed, among others. These methods, though they can result in functional devices, preclude researchers from knowing where exactly the reactions are happening on the substrate. This makes it impossible to do multiple simultaneous tests. Also, these sensors are not as sensitive as they could be because there is no way to ensure that the gas is reacting with the interior of the tube.
To address these problems, the NIST group built upon another design using a sheet of aluminum oxide about the thickness of a human hair and perforated with millions of holes about 200 nanometers wide. With the nanosized pores serving as a mold, the researchers dipped the aluminum oxide sheet in a solution of tungsten ions, coating the interior of the pores and casting the nanotubes in place. After the nanotubes were formed, the team deposited thin layers of gold on the top and bottom of the aluminum oxide membrane to act as electrical contacts. View schematic of the nanotube sensor.
The sensor’s high sensitivity derives from its design, which ensures that any sensor response is the result of the gas interacting with the interior of the nanotube. The researchers also note that this same technique can easily be adapted to form nanotubes of other semiconductors and metal oxides so long as the ends of the nanotubes remain open.
* R. Artzi-Gerlitz, K. Benkstein, D. Lahr, J. Hertz, C. Montgomery, J. Bonevich, S. Semancik, M. Tarlov. Fabrication and gas sensing performance of parallel assemblies of metal oxide nanotubes supported by porous aluminum oxide membranes. Sensors and Actuators B: Chemical. Available online Nov. 11, 2008.
Media Contact: Mark Esser, mark.esser@nist.gov, (301) 975-8735 

Insights into Polymer Film Instability Could Aid High Tech Industries
 |
Crystallization (left) occurs as polymers harden into thin films, which are used widely in electronics technology. But when dewetting (right) also occurs, inhomogeneities in the film can degrade performance. NIST scientists found that temperature determines which process dominates film formation, and that keeping certain angles between crystallization fronts can largely prevent dewetting.
Credit: NIST
View hi-resolution image |
While exploring the properties of polymer formation, a team of scientists at the National Institute for Standards and Technology (NIST) has made a fundamental discovery* about these materials that could improve methods of creating the stable crystalline films that are widely used in electronics applications—and also offer insight into a range of other phenomena.
The team has determined that temperature can play a decisive role in determining which of two competing processes—called crystallization and dewetting—will “take the lead” when a semicrystalline polymer film hardens, thereby granting qualitatively different properties to the finished film. The findings could lead to better control of these two processes, which can cause imperfections in polymer films during their formation.
Such imperfections can hinder the performance of potential new technologies, such as solar cells or thin film transistors, that employ organic polymer films on their surfaces, according to research chemist Christopher Soles. At this point, he said, the organic semiconductor industry is being hindered by a lack of understanding of crystal formation in thin polymer films.
“If organic photovoltaics—to take just one example—are ever to be realized and marketed, we need to understand how the film formation process works,” said Soles. “You have to know the properties of these materials first in order to control their stability.”
As a polymer film cools, two different things can happen locally within it: Either its molecules can crystallize, starting from some nucleation center (such as a scratch) and then expanding into the surrounding unstable film. Or, because of chemical differences between the polymer and its underlying substrate, such as a silicon wafer, the film’s molecules can “dewet”—similar to the beading up of water droplets on a windshield. If crystallization and dewetting occur simultaneously, they can couple to create imperfections that can be a nuisance for applications that rely on film uniformity, such as organic solar cells. The challenge is to bring these instabilities under control for constructive purposes.
Using model polymers with well understood crystallization behavior, the team discovered that a few degrees’ variation in temperature controls whether crystallization or dewetting will dominate the hardening process. They also found that when two growing crystals expand and collide, the stress created where they contact each other can cause dewetting—but if the angle between the two expansion fronts is small enough, then this localized dewetting might be averted.
“What’s cool about this discovery is not just that we have better understanding of polymer films, which are widely used in a range of coating and interface technologies,” said coauthor Jack Douglas, also of NIST’s polymers division. “There’s a whole class of mathematical problems in which you have multiple effects that are all fighting for domination of some field of action—the spreading of languages, for example, or the growth of different tissues within organisms. It’s a very common phenomenon, and this research could provide theoretical insight into those problems as well.”
Funding for this study was provided by the National Research Council.
* B.C. Okerberg, B.C. Berry, T.R. Garvey, J.F. Douglas, A. Karim and C.L. Soles. Competition between crystallization and dewetting fronts in this polymer films. Soft Matter, November 2008, 1-7. DOI: 10.1039/b806074f
Contact: Chad Boutin, boutin@nist.gov, (301) 975-4261 

Simply Weird Stuff: Making Supersolids with Ultracold Gas Atoms
 |
Artistic rendition of a supersolid made from two different types of ultracold atoms. The atoms are arranged in a regularly repeating pattern like a solid, but also can move frictionlessly like a superfluid. Yellow shape represents the electrical forces that the atoms feel, which vary in a regular pattern. Correspondingly. the density of the atoms (represented by the thickness of the spheres) also varies in a periodic fashion.
Credit: Ludwig Mathey, NIST/JQI
View hi-resolution image |
Physicists at the Joint Quantum Institute (JQI) of the National Institute of Standards and Technology (NIST) and the University of Maryland have proposed a recipe for turning ultracold “boson” atoms—the ingredients of Bose-Einstein condensates—into a “supersolid,” an exotic state of matter that behaves simultaneously as a solid and a friction-free superfluid. While scientists have found evidence for supersolids in complex liquid helium mixtures, a supersolid formed from such weakly interacting gas atoms would be simpler to understand, potentially providing clues for making a host of new “quantum materials” whose bizarre properties could expand physicists’ notions of what is possible with matter.
First theorized in 1970, a supersolid displays the essential characteristics of a solid, with atoms arranged in regularly repeating patterns like that of a crystal lattice, and of a superfluid, with the particles flowing frictionlessly and without losing any energy. Able to exist only at low temperatures, a supersolid behaves very differently from objects in the everyday world.
“If you add more clothing to a spinning washing machine, you increase the mass of its rim, and the machine needs to exert a greater force to make the wheel reverse direction,” explains lead author Ludwig Mathey. “But in a supersolid washing machine, some of the clothes would mysteriously hover in space, staying stationary as the washer spins and making it easier for the wheel to reverse direction. Moreover, these hovering, frictionless clothes would form a predictable pattern—such as frictionless socks alternating with frictionless shirts—just as atoms arrange themselves in a repeating pattern in a crystal.”
In 2004, Moses Chan and Eun-Seong Kim of Pennsylvania State University published a groundbreaking experiment on helium at low temperatures and gathered evidence for a supersolid phase. However, the interpretation of their observations has considerable uncertainties due to the complex nature of the particular system used in their experiments.
Now physicists Ludwig Mathey, Ippei Danshita and Charles Clark have identified a technique for making a simpler-to-understand supersolid, using two species of ultracold atoms confined in an optical lattice, a “web of light” that traps atoms in regular positions. In a paper* to be published in Physical Review A, the JQI team identifies conditions under which a cloud of ultracold atoms of two species (such as rubidium and sodium, or two slightly different forms of rubidium) can spontaneously condense into a state in which there is crystalline structure in the relative positions of atoms, e.g. a chain in which the two different types of atoms alternate regularly, but in which the entire cloud exhibits the frictionless, superfluid properties of a Bose-Einstein condensate (BEC). This remains hard to visualize in familiar terms—the accompanying image shows an artist’s conception of it—but the team identified clear experimental signatures (essentially photographs of the cloud), which could verify the simultaneous existence of these two seemingly incompatible properties.
The underlying technologies of optical lattices and Bose-Einstein condensation were pioneered at NIST and have sparked a renaissance in atomic physics with applications to NIST’s fundamental measurement missions, such as time and frequency standards and improved sensors of magnetic and gravitational forces. The supersolid is an example of a further direction of research in ultracold atomic physics: the design of quantum materials with fundamental properties not previously found in familiar matter.
* L. Mathey, I. Danshita and C. W. Clark, Creating a supersolid in one-dimensional Bose mixtures. Physical Review A. Published as a Rapid Communication on Jan. 12, 2009.
Media Contact: Ben Stein, bstein@nist.gov, (301) 975-3097 

Technology Innovation Program to Fund New Infrastructure Research
The National Institute of Standards and Technology (NIST) has announced nine awards for new research projects to develop advanced sensing technologies that would enable timely and detailed monitoring and inspection of the structural health of bridges, roadways and water systems that comprise a significant component of the nation’s public infrastructure. The awards, the first to be made under NIST’s new Technology Innovation Program (TIP), initiate up to $88.2 million in new research over the next five years on structure monitoring and inspection technologies, $42.5 million of it potentially funded by TIP.
The new TIP projects target innovative, low-cost and reliable sensors and related technologies that provide quantitative assessments of the structural integrity or degree of deterioration of bridges, roads, water mains and wastewater collection systems. The United States has 1 million miles of water mains, 600,000 bridges and 4 million miles of public roadway, and experts have pointed to serious gaps in the nation’s ability to monitor these networks adequately to ensure timely maintenance and repair. Twenty-five percent of U.S. bridges were rated as structurally deficient or functionally obsolete in 2007, according to the Federal Highway Administration, and the Environmental Protection Agency reported that there are 240,000 water main breaks per year in the United States. Baltimore, Md., as an example of an older urban area, suffered almost 1,200 water main breaks in 2003. Leakages and breaks in water distribution systems are estimated to waste up to 6 billion gallons of drinking water each day.
Damaged infrastructure also directly affects large numbers of Americans. The American Society of Civil Engineers estimates that Americans spend $54 billion each year in vehicle repairs caused by poor road conditions.
TIP supports, promotes and accelerates innovation in the United States through high-risk, high-reward research in areas of critical national need. The merit-based competitive program can fund cost-shared R&D projects by single small-sized or medium-sized businesses and joint ventures that also may include institutions of higher education, non-profit research organizations and national laboratories. TIP awards are limited to no more than $3 million total over three years for a single company project and no more than $9 million total over five years for a joint venture.
For additional details and a list of the nine selected research projects, see “NIST Technology Innovation Program Announces New R&D Projects to Develop Infrastructure Monitoring and Inspection Technologies.”
Media Contact: Michael Baum, michael.baum@nist.gov, (301) 975-2763 

Sorting Diamonds from Toothbrushes: New Guide to Protecting Personal Information
Thefts of personally identifiable information (PII), such as social security and credit card account numbers, are increasing dramatically. Adding to the difficulty of fighting this problem, organizations often disagree on what PII is, and how to protect it. Now, in a first-of-its-kind publication, the National Institute of Standards and Technology (NIST) has issued a draft guide on protecting PII from unauthorized use and disclosure.
“You can’t protect PII unless you can identify it,” says NIST’s Erika McCallister, a co-author of the new work. The new NIST publication provides practical guidelines for implementing a basic definition of PII established by the government’s Office and Management and Budget (OMB) in a 2007 memo: “information which can be used to distinguish or trace an individual’s identity”* either all by itself—such as fingerprints, which are unique—or in combination with other information, such as date of birth, which can belong to multiple people but can be narrowed down to an individual in connection with other data.
Echoing former national security advisor McGeorge Bundy, who once stated, “If we guard our toothbrushes and diamonds with equal zeal, we will lose fewer toothbrushes and more diamonds,” McCallister and her co-authors observe that, “All PII is not created equal.” A telephone area code holds less specific information about an individual than a social security number, so “you don’t need to protect things the same way,” McCallister says.
The NIST team recommends tailoring safeguards to the level of risk involved in holding personal information. PII should be graded by “PII confidentiality impact level,” the degree of potential harm that could result from the PII if it is inappropriately revealed. For example, an organization might require appropriate training for all individuals who are granted access to PII, with special emphasis on moderate- and high-impact PII, and might restrict access to high-impact PII from mobile devices, such as laptops and cellphones, which are generally at greater risk of compromise than non-portable devices, such as desktop computers at the organization’s headquarters.
The publication also recommends basic actions that organizations should take: identify all the PII they maintain, minimize the amount of PII they collect to what is strictly necessary to accomplish their mission, and develop incident response plans to handle breaches of PII. Such plans would include elements such as determining when and how individuals should be notified, and whether to provide remedial services, such as credit monitoring, to affected individuals.
The publication is intended primarily for U.S. federal government agencies, which must implement certain requirements on handling and protecting PII, but is intended to be useful to other organizations. The publication, known as Special Publication (SP) 800-122, “Guide to Protecting the Confidentiality of Personally Identifiable Information (PII),” is available at the NIST Computer Security Resource Center's draft publication Web page: http://csrc.nist.gov/publications/PubsDrafts.html#800-122.
* OMB M-07-16, “Safeguarding Against and Responding to the Breach of Personally Identifiable Information,” www.whitehouse.gov/omb/memoranda/fy2007/m07-16.pdf
Media Contact: Ben Stein, bstein@nist.gov, (301) 975-3097 

Quick Links
Seeking Excellence? Baldrige Conference Fulfills Your ‘Quest’
Learn about the exceptional practices and results of the 2008 recipients of the Malcolm Baldrige National Quality Award at the Quest for Excellence (QE) XXI, April 20-22, 2009, at the Hilton Washington in Washington, D.C. The 2008 Baldrige Award recipients are: Cargill Corn Milling North America (manufacturing), Iredell-Statesville Schools (education) and Poudre Valley Health System (health care).
This year’s QE conference will introduce a number of new features and sessions. For example, two-time Baldrige Award recipient The Ritz-Carlton Hotel Company LLC is teaming with conference organizers to offer QE XXI attendees an opportunity to experience the company’s “Community Footprints” program for social responsibility and give something back to the D.C. community. Participants will volunteer four hours of their time during QE XXI at Boys’ Town Washington, D.C. Other innovations at QE XXI include open microphone sessions for organizations to share their Baldrige-based best practices, a Linkedin online forum, more networking opportunities than previous conferences and “greener” operations during the three-day event.
Throughout the three-day conference, senior leaders and others from each of the 2008 Baldrige Award recipients and former recipients will give presentations and answer questions on their processes, tools and results in areas such as leadership, strategic planning, and customer and employee satisfaction. Two pre-conference workshops on April 19 will help attendees better understand how to use the Baldrige criteria for innovation and performance excellence as a tool to assess and improve their organization.
For more information and to register online, go to www.baldrige.nist.gov/Quest_for_Excellence.htm 
Bertocci, Kirchhoff Honored as AAAS Fellows
National Institute of Standards and Technology (NIST) alumni researchers Ugo Bertocci and William H. Kirchhoff were recently elected as 2008 fellows of the American Association for the Advancement of Science (AAAS), an honor bestowed upon AAAS members by their peers. The AAAS is the world’s largest general scientific society and publisher of the journal Science.
The two scientists are among 486 AAAS members cited “for meritorious efforts to advance science or its applications.” Bertocci is recognized for “pioneering contributions to the spectroelectrochemical and theoretical studies of metal surfaces and corrosion properties” while Kirchhoff receives his acknowledgment for work at the Department of Energy (in between tenures at NIST) “as an administrator of a major federal program of basic research in chemical physics and computational chemistry.”
New fellows will be honored on Feb. 14 at an event during the 2009 AAAS Annual Meeting in Chicago, Ill. For more information and the full list of the 2008 AAAS Fellows, go to www.aaas.org/aboutaaas/fellows/new_fellows.shtml. 
|

