Justification
Authorizing Legislation: Section 301 of the Public Health Service Act, as amended.
Budget Authority
| FY 2004 Actual |
FY 2005 Appropriation |
FY 2006 Estimate |
Increase or Decrease |
| FTEs |
BA |
FTEs |
BA |
FTEs |
BA |
FTEs |
BA |
| 376 |
$1,024,598,000 |
379 |
$1,051,990,000 |
379 |
$1,057,203,000 |
(0) |
$5,213,000 |
This document provides justification for the Fiscal Year 2006 activities of the National Institute on Aging (NIA), including HIV/AIDS activities. A detailed description of the NIH-wide Fiscal Year 2006 HIV/AIDS activities can be found in the NIH section entitled “Office of AIDS Research (OAR).”
Introduction
The mission of the NIA is to improve the health and well being of older Americans through an extensive program of high-quality research. There are currently 35 million Americans over the age of 65 B more than at any other time in history. Of these, more than four million are over 85, and some 65,000 have attained their hundredth birthday. In the coming years, the ranks of American elders are expected to swell; by 2030, the number of individuals age 65 and older likely will double, reaching 70.3 million and comprising a larger proportion of the entire population, rising from 13 to 20 percent.[1] In particular, explosive growth is anticipated among those most at risk for disease and disability, people age 85 and older, whose ranks are expected to grow from 4.3 million in 2000 to at least 19.4 million in 2050.
The aging of the population presents a number of social and economic challenges as increasing numbers of Americans reach retirement age. It also has important implications for our nation's health. For example, more than half of all Americans over age 65 show evidence of osteoarthritis in at least one joint.[2] Over half of Americans older than 50 have osteoporosis or low bone mass.[3] Cardiovascular disease, cancer, and diabetes remain common among older Americans, and as many as 4.5 million Americans suffer from Alzheimer's disease (AD).[4]
However, we now know that aging itself is not the cause of disease, disability, and frailty. Rather, disease and disabling processes influenced by age-related changes in the body and by unhealthy choices and sedentary lifestyles are the most important factors in compromising the quality of life for older people. This fundamental shift in thinking was reinforced most recently with insights from the National Long Term Care Survey (NLTCS). According to this study, the rate of disability among older Americans dramatically declined from the 1980s through the mid 1990s, even among people age 85 and older. These findings, along with evidence from a number of other studies, suggest more strongly than ever that disease and disability can be delayed or even prevented through specific interventions.
At the same time, however, this downward trend in disability among the elderly is in real danger of reversal. Data from the National Health Interview Survey have found that, over the same period, the disability rate actually rose significantly for people ages 18–59, with the two most important causes of disability being musculoskeletal problems, particularly back problems, and mental illness. Findings also indicated that combined disability cases from musculoskeletal problems and diabetes, both of which can be associated with obesity, were increasing more rapidly by the mid-1990s than those from other problems, and that the growing prevalence of obesity is the dominant factor in the rise in disability among individuals ages 50–59.[5]
The NIA portfolio emphasizes research aimed at increasing the Ahealthspan,”or years of healthy, active life expectancy. With guidance from the National Advisory Council on Aging, the NIA conducts and supports research on the biochemical, genetic, and physiological mechanisms of aging in humans and animal models; the structure and function of the aging nervous system; social and behavioral aspects of aging processes and the place of older people in society; and the pathophysiology, diagnosis, treatment, and prevention of age-related diseases, degenerative conditions, and disabilities. In all of its efforts, the Institute pays special attention to reducing health disparities among different groups of Americans. NIA-supported researchers can be found in all fifty states, and the Institute also conducts a thriving program of training opportunities for researchers wishing to become involved in aging research.
NIA and the NIH Roadmap
Through a series of broadly based but well-integrated initiatives that address the need to advance our understanding of the complexity of biological systems; to explore new organizational models for team science; and to conduct even more efficiently the complex clinical studies needed to make rapid medical progress, the ultimate goal of the NIH Roadmap for Medical Research is to accelerate medical discovery and improve people's health. A number of the NIH Roadmap initiatives are particularly relevant to aging research. For example, the “Molecular Libraries and Imaging” component of the Roadmap will offer biomedical researchers access to small molecules that can be used as chemical probes—providing new ways to explore the functions of genes, cells, and biochemical pathways in healthy aging and disease. Small molecule development, by providing chemical compounds to validate new drug targets, is crucial to the development of drugs for a variety of age-related diseases, degenerative conditions, and disabilities. The refinement of molecular imaging techniques, particularly those for imaging brain function, can similarly be accelerated by enhancing the development and availability of small molecule libraries, and could, in turn, greatly enhance our ability to diagnose and monitor neurological conditions such as Alzheimer's disease.
Another major theme of the NIH Roadmap is “Re-engineering the Clinical Research Enterprise.” Clinical trials are necessary to the development of new treatments for age-related conditions, but many aspects of patients' subjective experiences—such as symptom severity and frequency, emotional and social well-being, and perceived level of health and functional ability—are not adequately captured by conventional clinical and functional measures of disease status, even though they are important targets for treatment interventions. Correctly measuring patient-reported outcomes can be particularly challenging with regard to the ways in which chronic diseases and their treatments affect the elderly. One Roadmap initiative has established a network of investigators to improve the measurement of patient-reported outcomes from a diverse population of individuals, having a variety of chronic diseases. Ongoing projects of particular relevance to the aged population are addressing pain, fatigue, arthritis, psychiatric symptoms, including depression, and social functioning.
Alzheimer's Disease and the Neuroscience of Aging
Alzheimer's disease (AD) is the most common cause of dementia among people age 65 and older, and is a major public health issue for the United States because of its enormous impact on individuals, families, the health care system, and society as a whole. Scientists now estimate that as many as 4.5 million people currently suffer with the disease, and this number is expected to increase to 13.2 million persons by 2050, an almost threefold increase.[6]
People with AD gradually suffer memory loss and a decline in thinking abilities, as well as major personality changes. These losses in cognitive function are accompanied by pathologic changes in the brain, including the buildup of insoluble protein deposits called amyloid plaques and the development of neurofibrillary tangles, which are abnormal collections of twisted protein threads found inside nerve cells. Such changes result in death of brain cells and breakdown of the connections between them. AD advances gradually but inexorably, from early, mild forgetfulness to a severe loss of mental function called dementia. Eventually, people with AD become dependent on others for every aspect of their care. A diagnosis of AD is also associated with a sharply reduced lifespan; for example, the overall median survival for 70-year-olds in the United States is 15.7 years for women and 12.4 years for men, but a recent study found that this drops to 8.0 years and 4.4 years, respectively, for women and men with AD.[7]
The Genetics of Alzheimer's Disease
To date, only four of the approximately 30,000 genes in the human genome have been conclusively shown to affect the development of AD pathology. Three genes, amyloid precursor protein (APP), presenilin (PS) 1, and PS 2, are linked to the early-onset form of familial AD, which accounts for only a small percentage of all AD cases. A form of a fourth gene, APOE-ε4, which occurs in about one-fourth of the population, is a risk factor gene for late-onset AD (LOAD), and about half the AD cases have the ε4 form of the APOE gene. Geneticists have suggested that as many as four additional and as yet unidentified genes, at least one of which may be located on a specific region of chromosome 10, may be risk factor genes for LOAD. Finding new risk factor genes will help to identify pathways affecting the development or progression of AD, which can be become potential targets for treatment interventions.
Changes in Gene Activity in Early Aging and Alzheimer's Disease. Analysis of gene activity in discrete brain regions can provide important insights into the activities that underlie both normal brain aging and the pathological processes of neurodegeneration. Two recent studies demonstrate the utility of this approach. In one, investigators concluded that accelerated DNA damage may contribute to reduced gene expression, particularly the activity of genes involved in learning, memory and neuronal survival, and initiate a program of brain aging that starts early in adult life—in this case, around age 40. In a separate study, researchers show widespread changes in the genomic regulation of multiple pathways that involve the overactivity of tumor suppressor genes, as well as in genes involved in the differentiation of cells associated with myelinated axons. This suggests a provocative, but plausible, new model of AD pathogenesis that could account for the characteristic progression of AD throughout the brain along myelinated axons.
Genetic Variations Among Individuals Influence the Severity of Alzheimer's Disease. Insulin degrading enzyme (IDE) degrades amyloid β, a major component of amyloid plaques. In a recent study, the stretch of genetic material on chromosome 10 that contains the IDE gene and two other nearby genes was assessed for changes at a single point in the genetic code—known as a single nucleotide polymorphisms or SNP—and for haplotypes, which are stretches of DNA inherited in common among groups of people, in AD and control samples. Quantitative measures that are important to AD diagnosis and severity were also clinically assessed. Data strongly indicated the presence of alleles (alternative forms of a gene) and haplotypes that confer risk for AD within this region, and suggested that genetic variation within or extremely close to the IDE gene impacts both disease risk and traits related to the severity of the disease. Implementation of this approach on a broader scale is likely to be an effective tool in genetic analysis of complex diseases.
Early Diagnosis of AD
Early diagnosis of AD would be beneficial. For patients and their families, a definitive early diagnosis provides the opportunity to plan and pursue options for treatment and care, while the patient can take an active role in decisionmaking. For clinicians, accurate early diagnosis facilitates the selection of appropriate treatments, particularly as new interventions are developed. For researchers, earlier and more accurate diagnosis facilitates clinical studies of new therapies and preventive measures by allowing early and more targeted intervention, before cognitive loss becomes significant. Research suggests that the earliest AD pathology begins to develop in the brain long before clinical symptoms yield a diagnosis, and scientists are searching for reliable, valid, and easily attainable biological markers that, along with genetic, clinical, and neuropsychological assessments, could identify cases very early in the course of disease.
Abnormal PET Scans in Young Adults at Genetic Risk for Alzheimer's Disease. Using positron emission tomography (PET), patients with AD typically show decreased glucose metabolism, which correlates with brain activity, in specific brain regions. Other studies have shown decreases in metabolism in these same brain regions in nonsymptomatic middle-aged people who are at risk for AD. In a recent study, investigators extended these findings to show that cognitively normal people who are APOE ε4 carriers show the characteristic decreases in brain metabolism while in their 20s and 30s—decades before the possible onset of symptoms, and considerably earlier than previously recognized. These findings provide direct evidence that pathologic changes in the brains of APOE ε4 carriers can be seen many years before the onset of detectable cognitive decline and are consistent with the idea that AD may develop over decades. Many experts believe that the degeneration leading to AD should be treated as early in the course of the disease as possible, so as promising drug treatments develop, it will be even more important to identify those at risk and make early diagnoses.
Imaging Amyloid in the Living Brain. Until recently, there have been no imaging techniques that could effectively visualize characteristic AD pathologic features in the living human brain. However, investigators have developed a compound, Pittsburgh Compound B (PIB), that binds to brain amyloid and enables it to be imaged using PET. Another compound currently under development, IMPY, has shown promise in imaging amyloid plaques using single photon emission computerized tomography (SPECT) in a mouse model of AD. Although further research is needed, PIB, IMPY, and related compounds may ultimately play an important role in AD diagnosis, as well as studies of the pathology and course of the disease and evaluation of drug therapies targeted against amyloid deposits.
Changes in Cognitive Performance Over Time in People at Risk for AD. Scientists tested cognitively intact individuals, ages 48–77, on several components of memory over a two year period. APOE ε4 carriers 60 and older showed a significantly greater decline over time in new learning of a list of words, as compared with noncarriers. No difference in verbal learning ability was seen between carriers and noncarriers younger than 60 years old. These results suggest that longitudinal assessment of new learning may be a sensitive measure for detecting early cognitive changes in presymptomatic people who are at risk for AD.
Preventing Alzheimer's Disease
No intervention has been proven to prevent AD or even delay its onset, but scientists continue to seek risk and preventative factors for the disease, as interventions that impact the effect of a risk or preventative factor could potentially delay the onset of the disease or prevent it altogether.
Diabetes and Decline in Cognitive Function. Diabetes mellitus (DM) affects about one in five persons over age 60 years, [8] and it has been associated with a variety of adverse health effects. Recently four large-scale studies—the Religious Orders Study (ROS), the Nurses' Health Study (NHS), the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial, and the Rancho Bernardo Study (RBS)—linked DM to changes in cognitive function. The studies suggested the following: women with DM have an increased risk of developing substantial cognitive decline (NHS, MORE Trial, RBS); postmenopausal women whose blood glucose levels are elevated, but not yet in the “diabetic” range, i.e., “prediabetic,” are also at risk for significant cognitive impairment (MORE Trial); and oral hypoglycemic agents may ameliorate the increased risk in women (NHS, RBS). One study (ROS) suggested that men and women with DM have an increased risk of developing AD, and that, for both sexes, DM affects different cognitive systems differently. Together, these results indicate that a successful public-health prevention strategy for DM may also have major consequences for preventing or delaying AD. They further suggest that patients with DM who receive treatment for their condition may receive some protection from cognitive decline, in addition to the therapeutic benefit for DM.
Abnormalities in Lipid Metabolism in Nerve Cells Linked to AD. The pathogenesis of AD is tightly linked to amyloid beta (Aβ) deposition and oxidative stress, the cellular damage caused by free radicals, which are byproducts of normal cellular metabolism. However, it remains unclear how these factors result in dysfunction and death of brain cells. In a recent study, NIH researchers measured amounts of different lipids in brain cells from AD and control patients and found that AD patients had much higher levels of cholesterol and a lipid called ceramide specifically in brain regions important for learning and memory. These increases were associated with increased damage to nerve cells caused by free radicals. When cultured nerve cells were exposed to Aβ, similar overproduction of cholesterol and ceramide occurred. The increases were prevented and the nerve cells were protected when they were treated with the antioxidant vitamin E or a drug that prevents the accumulation of ceramide. These findings suggest a model of AD development that involves the disturbance of ceramide and cholesterol metabolism. This research further suggests that diets and drugs that target lipid abnormalities may be beneficial in the prevention and treatment of AD. Such drugs include cholesterol-lowering drugs, or statins, and earlier epidemiologic studies have shown a strong association between the use of statins and lower rates of AD. In January 2003, the NIA initiated the Cholesterol Lowering Agent to Slow Progression of AD (CLASP) study, a clinical trial to investigate the safety and effectiveness of the cholesterol-lowering drug simvastatin to slow the progression of mild to moderate AD. It is expected to be completed in November 2006.
Treating AD and Cognitive Impairment
To date, the Food and Drug Administration (FDA) has approved four medications for the treatment of mild to moderate AD symptoms. The first, tacrine (Cognex®), has been replaced by three newer drugs— donepezil (Aricept®), rivastigmine (Exelon®), and galantamine (Reminyl®). In 2003, the FDA approved memantine (NamendaTM), the first drug to treat moderate to severe AD. These drugs improve some patients' ability to carry out activities of daily living, help with behavioral symptoms, such as delusions and agitation, and can also maintain thinking, memory, and speaking skills for a period of time. However, none of these drugs can stop or reverse the disease process, and they appear to help only some patients and only for a period of months to a few years. Finding a truly effective intervention will depend on research progressing on a number of fronts, both in model systems and humans, and a major clinical research focus lies in testing the effectiveness of therapies in people without symptoms or who have only slight memory problems.
Vaccine That Removes Plaques May Also Remove Tangles. One way to treat AD successfully may be to interfere with early pathological changes in the brain, including the development of amyloid-β deposits (plaques) and the formation of tau-based neurofibrillary tangles, which are the hallmark pathological lesions of AD. Previous research has shown that in mice, immunization against Aβ can result in removal of amyloid plaques and maintenance of cognitive function. Now, researchers have found that a vaccine designed to clear amyloid plaques may also be effective against tau-based neurofibrillary tangles. Investigators injected “triple transgenic” mice, in which plaques, tangles, and AD-like brain damage are all present, with an antibody to Aβ. They found that Aβ was removed from both around and—unexpectedly—within neural cells. More surprisingly, they found that the antibody also removed early-stage accumulations of tau, though not late-stage tau lesions that resemble human neurofibrillary tangles. The reduction of tau pathology following an anti-Aβ treatment suggests a direct link between these two hallmarks of AD, and indicates that targeting the removal of Aβ early in the disease course might also eliminate tau pathology—thus removing or reducing both cardinal features of AD.
Donepezil May Have Short-Term Benefit for Mild Cognitive Impairment. The first NIH AD prevention trial, comparing the effects of vitamin E, donepezil (Aricept®), or placebo in preventing AD in people diagnosed with mild cognitive impairment (MCI), recently concluded at more than 70 sites across the U.S. The study is part of the Alzheimer's Disease Cooperative Study (ADCS) clinical trials consortium supported by NIA. Preliminary data from this study indicated that people with MCI taking donepezil, but not Vitamin E, were at reduced risk of progressing to AD for the first 18 months of the 3-year study when compared with their counterparts on placebo. The reduced risk of progressing from MCI to a diagnosis of AD among participants on donepezil disappeared after 18 months, and by the end of the 3-year period, the probability of progressing to AD was the same as that for the Vitamin E and placebo groups. However, among the subjects who did develop AD, those in the donepezil group experienced a statistically significant delay of almost 6-months in the development of AD compared to the placebo group.
Raloxifene May Reduce Risk of Cognitive Impairment in Postmenopausal Women. Studies of hormonal influences on cognitive aging in women have reported conflicting results, with some studies demonstrating a decreased risk for AD among users of hormone therapy and others, notably the Women's Health Initiative Memory Study (WHIMS), showing that postmenopausal women on certain regimens were actually at higher risk for cognitive decline. In a recent study—the Multiple Outcomes of Raloxifene Evaluation (MORE) trial—the selective estrogen receptor modulator (SERM) raloxifene (Evista®), frequently prescribed for the prevention and treatment of osteoporosis, appeared to reduce the risk of cognitive impairment in postmenopausal women. SERMs are compounds that mimic estrogen's actions in some tissues, but block the action of the body's naturally occurring estrogen in others. Raloxifene, like estrogen, promotes bone growth; however, it has antiestrogenic actions on the breast and uterus that reduce possible cancer-causing stimulation of these tissues postmenopause. Over 5,000 MORE participants were assigned to either 60 mg/day of raloxifene, or 120 mg/day of raloxifene, or placebo, and their cognitive function was assessed over the three years of the study. The researchers found that women taking 120 mg/day had a 33 percent lower risk of developing mild cognitive impairment (MCI), frequently a precursor condition to AD, than the other participants. They may also have a reduced risk of developing AD or other dementia, although this finding was not statistically significant. Risk of cognitive impairment did not differ between the 60 mg/day and placebo groups. Although extremely preliminary, these results suggest that treatment with raloxifene may offer women cognitive benefits, with fewer health risks than traditional hormone therapy.
People With Early AD Can Still Learn. In a recent study of cognitive rehabilitation among AD patients, who were taking medications to slow disease progression, subjects were randomly assigned to either a “cognitive rehabilitation” (CR) group or a control “mental stimulation” (MS) group. The subjects in the CR group participated in a series of biweekly sessions in which they were taught practical strategies for enhancing their ability to perform routine tasks such as face-name recognition, making change, and balancing a checkbook. The subjects in the MS group participated in activities that required memory, concentration, and problemsolving skills. At the end of the study, those in the CR group showed significantly improved ability to associate faces and names, had faster mental processing speeds, were better oriented to time and place, and were better able to make correct change for purchases than those in the MS group, although neither group showed memory improvement for manipulating objects or balancing a checkbook. The improvements were still evident three months after the intervention ended. These findings suggest that by combining specific cognitive rehabilitation strategies, people with AD can be helped to remain engaged in daily activities and retain a connection to their family and friends and the world as a whole for a longer period of time.
Exercise Plus Behavioral Management Improves Physical Function and Mental Health in AD Patients. Research has shown that even the oldest adults can improve cardiovascular functioning and increase flexibility, balance, and strength with systematic exercise training. However, it is unknown whether an exercise program would help reduce functional dependence and delay institutionalization among patients with AD. In a recent clinical trial, AD patients received either routine medical care or participated in an exercise plus behavioral management program. The exercise program consisted of aerobic/endurance activities, strength training, balance, and flexibility training. At three months, outcomes in the patients in the intervention group improved while routine care patients declined, and these differences remained up to two years later.
Caregiving of AD Patients
Most of the over four million Americans with AD today are cared for outside the institutional setting by an adult child or in-law, a spouse, another relative, or a friend. Caregivers frequently experience significant emotional stress, physical strain, and financial burdens, yet they often do not receive adequate support. Several recent studies have explored the problems faced by caregivers of AD patients, as well as possible interventions to reduce their burdens.
Sustained Benefit of Supportive Intervention for Depressive Symptoms in Caregivers of AD Patients. Family caregivers of relatives with Alzheimer's disease are at high risk for psychological distress, particularly clinical depression. This risk persists over the many years of caregiving and even after caregiving ends with the death of the care recipient. Investigators followed 406 participants in the NYU Spouse-Caregiver Intervention Study, a long-running study of an intervention for family caregivers of people with AD. Half of the spouse-caregivers received an initial period of intensive counseling, attended weekly support groups, and were encouraged on an ongoing basis to contact counselors for support. The second group of spouses was assigned to receive the “usual” support services for families of AD patients at the Center, which included information about resources and advice upon request, but no formal counseling.
When they began the study, the two groups showed comparable levels of depressive symptoms, but after one year, 29.8 percent of caregivers in the enhanced treatment group had symptoms of clinical depression compared with 45.1 percent of those in the usual care group. Significant differences between the two groups were found through the third year of followup. These results offer evidence that distress and depressive symptoms in family caregivers can be effectively eased and that the benefits can be sustained over a long period of time.
Long-Term Care Placement of Dementia Patients and Caregiver Health and Well-Being. In a recent study of the transition experience caregivers undergo when institutionalizing a relative, investigators found that race/ethnicity, caregiver burden, and global cognitive function of the patient were important predictors of institutionalization. They also found that caregivers who reported that providing help to their relative made them feel more useful, needed, appreciated, and important were less likely to institutionalize the patient. After the patient was institutionalized, half of spouse caregivers and one-quarter of nonspouse caregivers reported visiting the care recipient at least once a day, and nearly all reported visiting at least once a week. Spouses reported higher levels of depression both before and after placement and more anxiety after placement than nonspouse caregivers. These findings suggest that although caregiver bereavement studies have shown that caregivers demonstrate recovery in response to the death of their loved one, there appears to be less benefit to the caregiver from institutionalizing the relative, possibly because the caregiving role is not wholly relinquished after institutionalization.
Initiatives: AD and the Neuroscience of Aging
Advances in neuroimaging have the potential to transform the way we predict, diagnose, monitor, and even treat mild cognitive impairment and AD. The NIA is currently developing an Alzheimer's Disease Neuroimaging Initiative, a longitudinal, prospective, natural history study of normal aging, mild cognitive impairment, and early AD to evaluate neuroimaging techniques (e.g., MRI, PET) and other potential biomarkers of the disease. Biomarkers may decrease the time and cost of clinical trials, which would increase the safety and efficiency of drug development. An important aspect of this initiative is that the clinical, imaging, and biological data and samples will be made available promptly to all qualified scientific investigators in academic as well as industrial research communities. The initiative is planned as a partnership among the NIA/NIH and several other private and government organizations.
The NIA is accelerating the pace of Alzheimer's disease genetics research with its AD Genetics Initiative, a major new program to speed the process of creating a large repository of DNA and cell lines from families with multiple AD cases. The goal of this initiative is to develop the resources necessary for identifying the remaining late-onset AD (LOAD) risk factor genes, associated environmental factors, and the interactions of genes and the environment. The AD Genetics Initiative will intensify sample collection and encourage data sharing by providing access to the repository to qualified investigators.
Reducing Disease and Disability
About 79 percent of people age 70 and older have at least one of these seven potentially disabling chronic conditions—arthritis, hypertension, heart disease, diabetes, respiratory diseases, stroke, and cancer.[9] Other chronic conditions can compromise the health and quality of life of older Americans, as well; for example, a recent study found that 11 percent of U.S. men and 10.2 percent of U.S. women over age 65, and fully 20 percent of Americans over age 85, have anemia. This condition, while usually treatable, is often under-diagnosed and can be associated with a number of adverse health outcomes.[10] The burden of such chronic conditions is felt not only by individuals, but also by families, employers, and the health care system. Research to improve understanding of the risk and protective factors for chronic disease and disability can lead to the development of effective prevention strategies.
Treatment and Prevention of Disease
An Asthma Drug Improves Heart Function and Prevents Further Damage in Rats With Heart Disease. The β-adrenoreceptors (β-ARs) receive and react to nerve impulses in certain tissues throughout the body. In the heart, there are at least three types of β-AR, each found primarily in a different type of cardiac tissue and each causing different effects when activated. Suppression of β1-adrenoreceptors through the use of “beta blockers” is a standard treatment for congestive heart failure, which currently affects around five million Americans,[11] while drugs that stimulate β2-ARs act as vasodilators and are commonly used in asthma inhalers. In addition, laboratory studies have shown that continuous stimulation of β2-ARs protects heart cells from premature death.
NIH researchers have achieved promising results using a β2-AR stimulator in a rat model of heart disease. After inducing heart attacks in rats, they treated one group with a beta blocker, metaprolol, while additional groups received continuous treatment with one of two β2-AR stimulators, either fenoterol or zinterol. A final (control) group received no treatment. After six weeks, the rats' heart function was assessed and heart tissue was examined. The researchers found that the β2-AR stimulators were more effective than metaprolol in preventing further cell damage and death. In addition, treatment with fenoterol or zinterol, unlike metaprolol, was actually associated with improved heart function in the diseased rats. These results suggest that β2-AR stimulators, already widely in use for the treatment of pulmonary disease, may also be effective in the treatment of congestive heart failure.
Extended Outpatient Rehabilitation improves independence after hip fracture. Hip fractures are common in the elderly and can have a devastating impact on the ability of older patients to remain independent. Despite standard rehabilitation, up to three-fourths of patients with hip fractures fail to regain their walking ability or functional status within six to twelve months of surgery. To determine whether additional rehabilitation would improve function following hip fracture in frail elders, researchers conducted a randomized controlled trial comparing extended outpatient rehabilitation that included resistance training, to the usual program of low intensity home exercise following surgery to repair a hip fracture. Men and women 65 years or older with a recent hip fracture (within 16 weeks of repair) were randomly assigned to either supervised physical therapy with whole-body progressive resistance exercise training or to a control group doing home exercise focusing primarily on flexibility. The outcome measures included physical performance tests, measures of functional status, and activities of daily living (ADL) over six months. Changes in physical performance and functional status over time were significantly better for the intervention group compared to the control group: patients in the intervention group showed greater improvements in muscle strength, walking speed, and balance than patients in the control group. These results indicate that extended outpatient rehabilitation with progressive resistance training improves physical function and mobility among frail elderly hip fracture patients. Compared to usual care for this patient population, this program promotes better return to prefracture function, reduces disability, and improves quality of life.
Early-Life Determinants of Late-Life Health
A number of studies have investigated early-life determinants of later life health. For example, researchers have investigated the association of high blood pressure (BP) and hippocampal atrophy (HA) among Japanese-American men participating in the longitudinal community-based Honolulu Asia Aging Study (HAAS). The hippocampus is an area of the brain that is critical to learning and memory, and is vulnerable to vascular damage. The investigators found that men who had had high midlife BP, but had never been treated, had an increased risk for later HA compared with never treated men with normal midlife BP. In another study, researchers studying a cohort of young and middle-aged adults from a semirural black and white community in Bogalusa, Louisiana, examined the association between carotid wall thickness and traditional cardiovascular risk factors measured since childhood. Increased arterial stiffness is a known predictor of cardiovascular-related diseases and death at middle and older ages, and carotid artery wall thickness is associated with cardiovascular risk factors and predicts atherosclerosis in middle- and older aged adults. They found that measures of LDL (“bad”) cholesterol and relative weight in childhood predicted carotid wall thickness in the adults, and that childhood blood pressure was a consistent predictor of arterial stiffness in adulthood. Recent research has pointed to a number of early life conditions that have far-reaching associations with a range of chronic conditions, including exposure to adverse conditions in utero, infectious diseases and environmental toxins, nutritional deficits, childhood poverty and stressful family conditions. Earlier identification of risk factors that are associated with diseases that manifest later in life could lead to the development of earlier and better preventive strategies.
Elevated Levels of Homocysteine May be an Important but Modifiable Risk Factor for Osteoporosis. To test the hypothesis that increased blood homocysteine levels may be a risk factor for osteoporosis, the relationship between circulating homocysteine levels and later hip fractures was evaluated in 825 men and 1174 women, ranging in age from 59 to 91 years, from whom blood samples had been obtained years earlier. After a followup of 12.3 years for men and 15.0 years for women, there was a significantly greater risk of hip fracture for both men and women with high homocysteine compared to those with low levels—risk was increased in men and women by a factor of 4 and 1.9 respectively. Because homocysteine levels can be modified by diets or vitamin supplements with sufficient levels of vitamins such as folic acid, B6 and B12, such dietary strategies could reduce the burden of hip fractures in older individuals.
Importance of Walking for Maintaining Mobility. In a recent study of community-dwelling women enrolled in the Women's Health and Aging Study, investigators found that functionally limited women ages 65 and older, who walked at least eight blocks per week outside their homes, were better able to maintain their functional capacity and walking ability than women who walked less or did not get out the door at all. This effect is independent of initial functional capacity, disease profile, health-related behaviors, and psychological and social-demographic factors. These results provide strong evidence that even a small amount of regular walking can help to maintain mobility.
Appetite and the Immune System: A New Model. Loss of appetite and decreased food intake are common among the seriously ill. Appetite regulation is complex and involves a number of factors; for example, appetite is suppressed by leptin, a protein found in fat cells, but stimulated by ghrelin, a recently identified hormone produced by stomach cells. There is also increasing evidence that the immune system is involved, with immune-based proteins known as inflammatory cytokines acting on the nervous system to control appetite. NIH researchers have recently found that, in addition to stomach cells, ghrelin is produced in certain immune cells, along with its receptor protein, GHS-R. When ghrelin binds to GHS-R, the result is inhibition of inflammatory cytokines associated with appetite loss. They further found that leptin increases cytokine activity, while also spurring increased expression of GHS-R by T-lymphocytes, a different type of immune cell. These findings provide a model of how ghrelin and leptin work together to control immune cell activation and inflammation with regard to the appetite, and also suggest that drugs that stimulate ghrelin/GHS-R may be useful in the management of wasting associated with chronic disease.
Biology of Aging
Aging is accompanied by gradual changes in most body systems. Research on the biology of aging focuses on understanding the cellular and molecular processes underlying these changes as well as those accompanying the onset of age-related diseases. As scientists learn more about these processes, experiments can be designed to understand when and how pathological changes begin, providing important clues toward developing interventions to prevent or treat disease. A great deal has been learned about structural and functional changes that occur in different body systems. Research has expanded our knowledge, too, of the biologic factors associated with extended longevity in humans and animal models.
Adult Mice Continue To Produce Eggs. One of the basic underpinnings of reproductive biology has been the tenet that the number of oocytes (eggs) in the ovaries of most mammals—including human women—is fixed at birth and declines throughout life, coinciding with a woman's diminishing fertility as she approaches menopause. However, NIH-supported researchers have recently uncovered surprising evidence that egg production in mice may continue on a small scale throughout life. While additional research is needed, the results of this study have called into question decades of scientific thought. The finding that new eggs are produced into adulthood in mice may, if extended to human women, lead to interventions to regulate the rate at which oocytes are formed. This could, in turn, have important implications on the treatment of premature ovarian failure, the extension of fertility, or even the timing of menopause.
Regeneration of Skeletal Muscle by Hematopoietic Stem Cells. As we age, the ability of our tissues to regenerate in response to injury diminishes, although the mechanisms of this decline are not known. Recently, research results have suggested that therapies that stimulate the body's naturally occurring stem cells may help in the repair or regeneration of muscle and possibly other tissues. In the first study, researchers found that satellite cells—adult stem cells found in muscle tissue—have an increasingly impaired ability to proliferate with increasing age, and that this is at least partly due to insufficient activation of a protein called Notch. They found that inhibition of Notch impairs muscle regeneration in mice, whereas activation of Notch restores regenerative potential to old muscle, suggesting that Notch is a key factor in muscle regeneration. In the second study, investigators found that a single hematopoietic stem cell is not only able to replenish components of blood, but can also participate in muscle regeneration in mice. Integration of bone marrow-derived cells into myofibers occurs spontaneously at low frequency and increases with muscle damage, suggesting that stem cell therapy might be effective in attenuating age-related muscle dysfunction.
Inherited Defects in Mitochondrial Function Contribute to Diabetes Risk in Children of Diabetics. Insulin resistance is a metabolic disorder that is thought to precede the development of type 2 diabetes, the most common type of diabetes in adults. The initial manifestations of insulin resistance are abnormally high levels of insulin, as well as levels of glucose in the blood that are higher than normal, but not severe enough to be classified as diabetes. Two important factors noted in the development of insulin resistance are age-related accumulation of fat in skeletal muscle cells and the dysfunction of mitochondria (cell components responsible for combining oxygen with cellular “fuels” such as fat and sugar to provide usable energy for body functions) in skeletal muscle. Insulin resistance also appears to be involved in the development of diabetes in the offspring of type 2 diabetics.
In a recent study, researchers found that insulin resistance in the young (primarily in their 20s and 30s), lean, offspring of type 2 diabetics was related to increased fat content of muscle and inherited defects in mitochondrial function, suggesting that these alterations may represent the earliest antecedent conditions in the development of type 2 diabetes. Additional studies of early metabolic changes contributing to insulin resistance and diabetes across various ages and within families, especially through the use of noninvasive methods, will be valuable in identifying metabolic problems at the earliest stages and to the development of effective primary prevention strategies for type 2 diabetes.
Extending the Lifespan
Identification of the factors that affect the overall lifespan of an organism will help us better understand the aging process, and will also help us develop interventions to keep older people healthy and free of disease and disability as long as possible. Over the last ten years, the NIA Longevity Assurance Gene (LAG) Initiative has been pivotal in the identification of multiple genes, pathways, and biological processes involved in the regulation of longevity and aging in multiple organisms (yeast, nematode, fruit fly, mouse, and human). Through the use of both invertebrate and mammalian models, the LAG Initiative has identified common factors and mechanisms that mediate longevity and extend health span.
Gene That Regulates Cholesterol Is Related to Exceptional Longevity. Exceptional human longevity tends to run in families. Both environmental and genetic factors may account for this. Previous genetic studies had indicated that variants of two genes that regulate blood cholesterol are found especially frequently in centenarians, suggesting that they contributed in some way to exceptionally long life. Researchers studying Ashkenazi Jewish centenarians and their children (whose average age was 68) found that a variant of a third gene that regulates cholesterol is found much more commonly in the centenarians and their children than in the general population. The variant causes the size of lipoproteins, the particles that carry cholesterol in the blood, to be larger than average (larger lipoproteins are less likely to cause the buildup of plaque along arterial walls), and is associated with high levels of high-density lipoprotein (HDL or “good” cholesterol), which tends to protect against cardiovascular disease. These results reinforce evidence that genetic regulation of cholesterol has important effects on longevity and that certain genetic variants predispose to exceptional longevity. In addition, this study, more than previous ones, provides a link between a particular gene and the mechanism by which it exerts its effects (regulation of HDL cholesterol by control of lipoprotein particle size).
Improved Screening for Interventions That Regulate Longevity. The discovery of genes and drugs that affect lifespan has been delayed by the fact that measuring an organism's lifespan is inherently time-consuming and requires a large number of animals. However, researchers have recently developed a much faster assay for interventions that slow down aging in the fruit fly. Using this new screen, life-extending mutations can be identified in only a few weeks. This assay can be used not only to facilitate identification of long-lived mutants, but also to identify pharmacological interventions that increase longevity; for example, using this assay, investigators found that the antioxidant lipoic acid, but not vitamin E, extends fruit fly lifespan.
Story of Discovery: Eat, Drink, and Live Longer? Caloric Restriction, Sirtuins, and Longevity. In 1513, the Spanish explorer Juan Ponce de León began his search for the fabled “fountain of youth,” a spring whose waters were said to confer eternal life and boundless good health upon anyone who drank from it. Although Ponce de León's quest was not a complete failure – he did discover the Florida peninsula – he did not, ultimately, find what he was looking for. The idea of a fountain of youth eventually receded into myth.
However, the idea of an intervention that may extend both life and health (for a while, if not indefinitely) has gained legitimacy over the past century. With extensive NIH support, researchers have been investigating both genetic and environmental influences on healthy aging and longevity, as well as the interplay between them. Recently, NIH-supported researchers have discovered a family of cellular enzymes that will, under certain conditions, retard cellular aging and delay cellular death. They have also identified at least one compound that activates these enzymes and that significantly extends the lifespan of several experimental models as a result – and they have demonstrated that this compound exists in a number of common foods. Although additional research is needed, scientists are optimistic that the ability to simultaneously extend the lifespan and safeguard health may not be a myth after all.
Ironically, the discoveries of this family of life-extending enzymes, called sirtuins, and the food-derived compound that stimulates them are rooted in studies of food deprivation. In 1914, eventual Nobel laureate Francis Peyton Rous published a paper showing that reduction of food intake retards carcinogenesis in rats. Although Rous's work did not directly address the question of longevity, he was the first of many researchers to demonstrate a clear health benefit from caloric restriction (CR), or reducing an organism's caloric intake while ensuring adequate intake of essential nutrients. In fact, later research would show that CR also slows progression of kidney disease and protects against autoimmune disease in rodent models. In 1935, the pioneering work of McCay et al. showed that CR rats lived much longer than others, and research in the 1940s and 1950s extended these findings to other species, including invertebrates such as yeast and worms, which are today a major source of important data on aging.
Research in this area continued over the next several decades, with a number of theories proposed as to how and why CR extends the lifespan. Investigators discovered that CR animals have low levels of circulating insulin and an increased daily peak in certain stress hormones, a finding that led them to propose a theory for how CR extends life: as a mild form of chronic stress, it may prepare organisms to cope more effectively at the physiologic level with more intense stresses, including age-related oxidative damage.
Meanwhile, a separate group, this one working with yeast, was beginning to uncover the biological mechanisms through which CR may function. In 1997, this NIH-supported team discovered that a gene called sir2, for silent information regulator, recognizes food deprivation within the cells—in yeast, at least—and sets into motion the changes that increase the organism's lifespan. When faced with a stressor (such as food deprivation) sir2 activates sirtuins, which prevent cell death by enhancing DNA repair processes and production of protective antioxidants. Research findings from last year suggest that similar mechanisms work in rodent and human cells, in which CR activates SIRT-1, the mammalian form of sir2. The case for sirtuins as key to CR's ability to extend life was also strengthened by the finding that CR does not extend life in animals that have been genetically altered to lack sirtuins.
Because an intensive regimen of restricted food intake may prove too difficult for most people to follow over the long term, investigators are now searching for compounds that mimic CR. Last year, NIH-supported researchers identified several compounds that increase both sir2 activity and longevity in yeast. The most potent activator of sir2 identified so far is resveratrol, a compound found in many foods—notably red wine, but also in grapes, mulberries, and peanuts. Resveratrol also appears to increase longevity in nematodes and fruit flies. The researchers theorize that sir2 expression and activity, as stimulated by resveratrol, mimic the mild stress induced in yeast by caloric restriction, essentially sending out a “false alarm” that tricks the cell into thinking it is starving and setting into motion the cell-protective events described above.
Whether such an intervention would work in mammals has not yet been tested, but diet supplementation with these compounds may be effective against a number of potentially debilitating conditions in humans. For example researchers have uncovered intriguing hints that sirtuins may affect the cells' capacity for fat storage, perhaps working through the insulin/IGF signaling pathway, which is known to influence lifespan in a number of lower organisms, including rodents. This would suggest that compounds that activate sirtuins and SIRT-1 may be useful in treating obesity and the problems associated with type 2 diabetes.
Ponce de León traveled all the way to Florida to seek the fountain of youth. Will tomorrow's intrepid explorer need venture only as far as his or her medicine cabinet—or kitchen? It's far too early to make such a prediction. For one thing, there have been no clinical studies of resveratrol or related compounds in humans; interventions that are effective in yeast and rats may be less effective, or have unexpected side effects, in higher organisms. Even if such compounds do work, it's hard to know exactly how many years they will add to the average human lifespan, although interventions that increase longevity in rodents typically do so by about 30 percent. Even CR itself remains to be scientifically validated as a useful intervention for healthy aging and longevity in humans, although research results to date have been somewhat promising. Compounds that stimulate sirtuin activity, whether ingested through a pill or through a nightly glass of cabernet, may one day be widely recommended as a way to increase the odds of enjoying a longer life accompanied by a longer period of good health.
Initiatives: Biology of Aging
The identification of “longevity genes” is complex and necessarily interdisciplinary, involving ongoing interactions between basic and epidemiologic researchers to accelerate discovery of, and confirm, translational findings. To facilitate identification and understanding of longevity genes, the NIA has formed the Longevity Consortium—a system for rapid generation, review, and funding of new projects. Components of the Consortium include multiple basic laboratories addressing relevant disciplines including cell and molecular biology, physiology, and biochemistry; diverse populations and large sample sizes to allow analyses of subgroups and covariates; registry and/or database capacity to allow rapid identification of possible cases and controls, and genotype and phenotype information; and genotyping, genomics, computational, and cell line repository facilities to allow standardization and economies of scale. The Consortium provides a system for rapid information exchange among basic and epidemiologic researchers to convey new findings and conduct follow-up studies, as well as for rapid review and funding to allow faster startup of basic or epidemiologic studies on new alleles of interest.
Members of the Consortium include epidemiologists, geneticists, population biologists, statisticians, and others with an interest in the genetic and molecular basis for longevity, and draws on the study populations of 15 of the largest human aging studies, including the Cardiovascular Health Study, the Women's Health Initiative, Health ABC, the Study of Osteoporotic Fractures, the Rotterdam Study, the Honolulu Heart Study, and the New England Centenarian Study. Altogether, Consortium researchers will have access to data on some 200,000 study subjects. Through this Consortium, it is expected that basic science discoveries will lead to tests of hypotheses in human populations, and observations in populations will, in turn, suggest tests of biologic mechanisms.
Behavioral and Social Research
Behavioral and lifestyle factors have a profound impact on health throughout the lifespan. For example, older adults can help to prevent disease and disability and improve their quality of life through healthy behaviors such as proper nutrition and exercise, use of preventive health care, and avoiding smoking and alcohol abuse. NIA research on behavioral and social factors in aging encompasses a number of areas, including economic implications of aging at both the personal and societal levels, the effects of behavior and attitude on health, and the demographics of aging.
The Role of Public “Report Cards” in Medical Markets. In recent years, public “report card” programs have been started by both private and public organizations to supply information regarding the quality of medical care provided by hospitals and physicians. By supplying patients and referring physicians with more information when making decisions about where to receive care, report cards can be advantageous. They can also provide hospitals with information that could be used as a guideline to improving care. However, report cards could also create problems. If the performance measures fail to account for the underlying health of the patients being treated, then the measure of performance might reflect more the existing health problems of patients served by a particular hospital than the actual quality of care being rendered. This could lead to flawed decisionmaking by patients and referring physicians based on poor data, as well as causing hospitals to become less willing to serve particularly high-risk patients in order to avoid having their reputations penalized. Recently, researchers used Cardiac Surgery Reporting System (CSRS) data from New York State to evaluate the impact of report cards on the distribution of where patients go for bypass surgery, and whether good or bad reports lead to improvements in the quality of care, measured by mortality. In their study, being reported as a poor performer (“high mortality”) was associated with a 10 percent reduction in bypass surgery patients per month in the 12 months following the report. The shift appeared to be primarily due to patients and referring doctors choosing to have procedures performed at low-mortality hospitals. However, the researchers found that hospitals flagged as poor performers improve patient mortality by 1.2 percentage points within the 12 month period following the report. Since this study took into account the severity of patient condition, the latter change does not appear to be simply due to sicker patients moving to low mortality hospitals. Indeed, the findings show that low-performing hospitals lose relatively healthy patients to competing facilities. While additional research is needed to identify exactly what mechanisms underlie the reported changes, the findings do provide evidence that report cards could have a beneficial impact on the quality of healthcare.
Health Disparities Research
The health status of racial and ethnic minority groups in the U.S. has improved steadily over the last century. Despite such progress, disturbing disparities in health persist between majority and minority populations. Demographic projections predict a substantial change in the racial and ethnic makeup of the older population, heightening the need to examine and reduce differences in health and life expectancy.
Assessing Cognitive Differences. Census data indicate that in the United States, Latinos have become the largest ethnic minority group, and it is important to understand their health needs. However, it may be difficult to assess the incidence of age-related conditions, particularly cognitive impairment, in this population, as many relevant neuropsychological instruments are inappropriate for studies of older Latinos. Last year investigators found that one test, the Spanish English Verbal Learning Test, is a valid and sensitive measure of cognitive functioning. More recently, the researchers determined that there may be a similar pattern of cognitive declines for verbal memory and expressive language and findings on brain imaging that predict declines in everyday functioning in both Hispanic and Caucasian older adults.
Racial Differences in Family Caregiving. Resources for Enhancing Alzheimer's Caregiver Health (REACH) is a unique, two phase, multisite research program sponsored by the NIA and the National Institute on Nursing Research to carry out social and behavioral research on interventions designed to enhance family caregiving for AD and related disorders. Recently, REACH investigators published two companion papers addressing the issue of racial differences in family caregiving. In one analysis, African-American caregivers reported lower anxiety, better well-being, less use of psychotropic medications, more benign appraisals of stress and perceived benefits of caregiving, and greater religious coping and participation than white caregivers. In the other study, Latina caregivers reported lower appraisals of stress, greater perceived benefits of caregiving, and greater use of religious coping than white caregivers. In addition, several differences emerged between less and more acculturated Latinas, emphasizing the need to examine heterogeneity among Latino caregivers.
The NIA is initiating a new project, “Promoting Research Participation Among Black and Hispanic Seniors.” Through data analyses, interviews of informed community members, and focus group discussions, this year-long project at the Yale-Older Americans Independence Center will identify characteristics of Black and Hispanic study participants and nonparticipants and develop recommended practices to improve and promote the recruitment and retention of Black and Hispanic older adults in aging-related research and studies of geriatric health conditions.
Innovations in Communications
NIHSeniorHealth.gov. Last year, the NIH launched NIHSeniorHealth.gov, a unique web site geared toward the health needs of older adults. Developed by the NIA and the National Library of Medicine, the content of this web site is easy for older persons to read, understand, remember, and navigate, using large print and short, easy-to-read segments of information repeated in a variety of formats—such as open-captioned videos and short quizzes—to increase the likelihood that it will be remembered. The site also has a Atalking@ function, allowing users the option of having the text read to them. The content focuses on health topics of particular interest to older people—AD and AD caregiving, arthritis, hearing loss, and several common types of cancer—and will be regularly expanded and updated.
In its first year, NIHSeniorHealth.gov was extremely successful, attracting some 380,000 unique visitors and garnering over three million page views. It was also one of six programs, and the only web site, to receive an “Industry Innovators Award” from the International Council on Active Aging. Recent innovations include a “printer-friendly” version of the online text, as well as more text sizing options. A “Share a Senior Exercise Story” feature, in which older adults will be invited to send in their stories and photos to serve as an inspiration to others, is planned, and a Spanish-language version of the site is also under development.
Meals on Wheels Initiative. During a 2002 Congressional hearing, it was recommended that NIA and the Administration on Aging (AoA) work together to disseminate research-based consumer education materials to the thousands of seniors who participate in the Meals-on-Wheels (MOW) program across the Nation. In participation with AoA, NIA conducted focus groups with the MOW Association of America to identify the types of information of greatest interest to MOW's clients and the best ways to deliver such information. Now, a new booklet entitled “Take Your Medicines the Right Way – Everyday!”, as well as a plastic cup with the same message, are being made available to MOW providers for their clients free of charge. The booklet is in easy-to-read language and covers important steps to help ensure safe and effective medication use.
Conclusion: Meeting New Challenges Through Aging Research
As our population rapidly grows older, it is ever more urgent that we find effective ways to address the often devastating diseases and conditions associated with advanced age. Since the NIA's founding in 1974, groundwork has been laid for today's important advances in understanding basic aging, preventing disease and disability, including AD, and defining special social and behavioral issues for older individuals, their families and caregivers, and clinicians. The latest studies provide additional basic understandings as well as improved interventions to treat, and even prevent, some of the more devastating and disabling aspects of aging. With such research continued and intensified, we can move forward in meeting the promise of a healthy old age by improving the health and well being of older people in America.
The NIH Neuroscience Blueprint
Overview
The Blueprint is a framework to enhance cooperation among fifteen NIH Institutes and Centers that support research on the nervous system. Over the past decade, driven by the science, the NIH neuroscience Institutes and Centers have increasingly joined forces through initiatives and working groups focused on specific disorders. The Blueprint builds on this foundation, making collaboration a day-to-day part of how the NIH does business in neuroscience. By pooling resources and expertise, the Blueprint can take advantage of economies of scale, confront challenges too large for any single Institute, and develop research tools and infrastructure that will serve the entire neuroscience community.
FY 2005
For fiscal year 2005, the Blueprint participants are developing an initial set of initiatives focused on tools, resources, and training that can have a quick and substantial impact because each builds on existing programs. These initiatives, with the participation of all Blueprint Institutes, include an inventory of neuroscience tools funded by the NIH and other government agencies, enhancement of training in the neurobiology of disease for basic neuroscientists, and expansion of ongoing gene expression database efforts. The NIA has been an active member in this initiative, and participates in the expansion of the ongoing Gene Expression Nervous System Atlas (GENSAT) and microarray consortium projects, as well as in the development of supplements to training grants to include course work on specific diseases of the brain. In addition, the NIA has a special emphasis upon the neurodegenerative diseases, such as Alzheimer's disease, which occur predominantly later in life. Current NIA initiatives, such as the Alzheimer 's Disease Neuroimaging Initiative, a multisite, longitudinal, prospective, naturalistic study of normal cognitive aging, mild cognitive impairment, and early Alzheimer's disease, can provide bases upon which Blueprint initiatives can develop.
FY 2006
Advances in the neurosciences and the emergence of powerful new technologies offer many opportunities for Blueprint activities that will enhance the effectiveness and efficiency of neuroscience research. Blueprint initiatives for fiscal year 2006 will include systematic development of genetically engineered mouse strains of critical importance to research on nervous system and its diseases and training in critical cross cutting areas such as neuroimaging and computational biology. The NIA will work with the other Blueprint Institutes on new initiatives such as the Neuromouse Project, which will investigate the function of each gene in the mouse brain, the training of physicians and scientists in translational opportunities in the neurobiology of disease, and the development of neuroscience resource cores to provide regional centers for access to specialized technologies for research on the brain. The NIA will also continue to work with NINDS and NIMH on the Cognitive and Emotional Health Project part of the Neuroepidemiology Initiative.
Budget Policy
The Fiscal Year 2006 budget request for the NIA is $1,057,203,000, an increase of $5,213,000 and 0.5% over the FY 2005 Appropriation. Also included in the FY 2006 request, is NIA's support for the trans-NIH Roadmap initiatives, estimated at 0.89% of the FY 2006 budget request. This Roadmap funding is distributed through the mechanisms of support, consistent with the anticipated funding for the Roadmap initiatives. A full description of this trans-NIH program may be found in the NIH Overview. NIA is participating in the NIH Neuroscience Blueprint. The Fiscal Year 2006 request includes $1,400,000 for a variety of Neuroscience Blueprint initiatives, including neuroscience cores, training initiative, and the Neuromouse project.
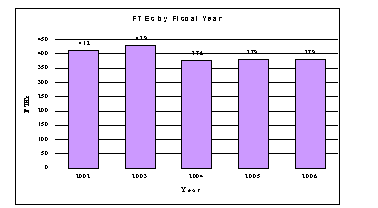
A five-year history of FTEs and Funding Levels for NIA are shown in the graphs below:
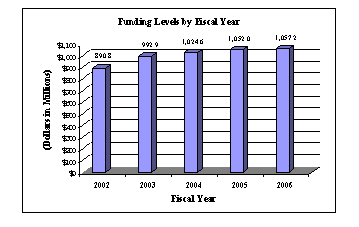
NIH's highest priority is the funding of medical research through research project grants (RPGs). Support for RPGs allows NIH to sustain the scientific momentum of investigator-initiated research while pursuing new research opportunities. The average cost of competing RPGs will be $400,000 in FY2006. While no inflationary increases are provided for direct, recurring costs in noncompeting RPG's, where the NIA has committed to a programmatic increase in an award, such increases will be provided.
Advancement in medical research is dependent on attracting, training, and retaining the best and the brightest individuals to pursue careers in biomedical and behavioral research. In the FY2006 request, most stipend levels for individuals supported by the Ruth L. Kirschstein National Research Service Awards are maintained at the FY2005 levels. To help prevent the potential attrition of our next generation of highly trained postdoctoral trainees, stipend levels for postdocs with 1–2 years of experience are increased by 4.0%. This will bring these stipends closer to the goal NIH established for postdoc stipends in March, 2000. In addition, individual postdoctoral fellows will receive an increase of $500 in their institutional allowance for rising health benefit costs. The need for increased health benefits is particularly acute for these postdoctoral trainees, who, because of their age and stage of life are more likely to have family responsibilities. The increases in stipends and health insurance are financed within the FY2006 request by reducing the number of Full-Time Training Positions, because NIH believes that it is important to properly support and adequately compensate those who are participating in these training programs, so that the programs can continue to attract and retain the trainees most likely to pursue careers in biomedical, behavioral and clinical research. NIA will support 541 pre- and postdoctoral trainees in full-time training positions.
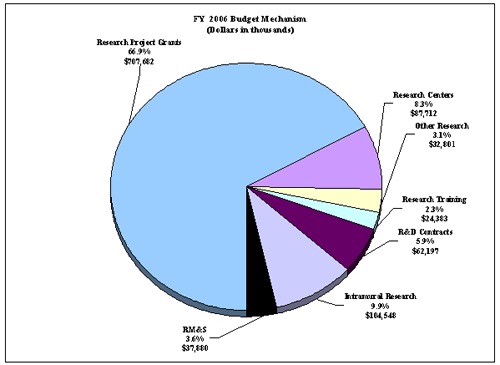
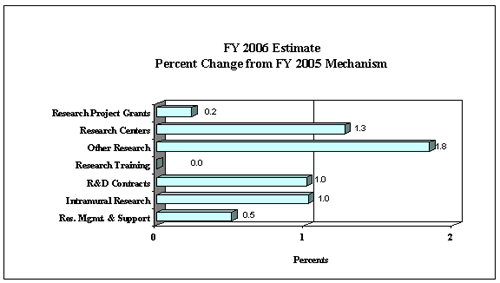
The Fiscal Year 2006 request includes funding for 76 research centers, 236 other research grants, including 206 clinical career awards, and 133 R&D contracts. Intramural Research will receive a 1% increase and Research Management and Support will receive an increase of 0.5 percent, the same as the NIH total increase.
The mechanism distribution by dollars and percent change are displayed below:
[1] Federal Interagency Forum on Aging Related Statistics. Older Americans 2000: Key Indicators of Well-Being. 2000.
Lakdawalla DN, Bhattacharya J, Goldman DP. Are the Young Becoming More Disabled? Health Affairs 23(1): 168-76, 2004.
[10] Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, and Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 104: 2263-2268, 2004.
[11] Data are from the National Center for Health Statistics. See Morbidity & Mortality: 2004 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Heart, Lung, and Blood Institute, 2004.
