Methods Development Team
Industrial Hygiene Chemistry Division
OSHA Salt Lake Technical Center
Sandy UT 84070 |
CONTENTS
EVALUATION GUIDELINES
PREPARATION OF WRITTEN REPORTS
LIST OF FIGURES
-
Figure 1 Evaluation scheme for OSHA chromatography methods
-
Figure 2 Example of plotted DLAP data
-
Figure 3 Example of a calibration curve
-
Figure 4 Example of breakthrough data
-
Figure 5 Example of plotted data to determine the recommended sampling time and sampling rate
-
Figure 6 Example of a storage test
-
Figure 7 Example of plotted DLOP/RQL data
-
Figure 8 Example of a calculated RQL when recovery is the determining factor
-
Figure 9 Plot of atmospheric pressure vs. elevation
-
Figure 1.2 Plot of data to determine the DLOP/RQL
-
Figure 3.5.1 Chromatogram obtained at the target concentration with the recommended conditions
-
Figure 3.5.2 Calibration curve of {analyte}
-
Figure 4.1 Plot of data to determine the DLAP
-
Figure 4.2.1 Plot of data to determine the DLOP/RQL
-
Figure 4.2.2 Chromatogram of the RQL
-
Figure 4.5.1.1 Ambient storage test for {analyte}
-
Figure 4.5.1.2 Refrigerated storage test for {analyte}
-
Figure 4.5.2.1 Ambient storage test for {analyte}
-
Figure 4.5.2.2 Refrigerated storage test for {analyte}
-
Figure 4.7.1 Five percent breakthrough air volume for {analyte}
-
Figure 4.7.2 Example of plotted data to determine the recommended sampling time and sampling rate
-
Figure 4.10 Mass spectrum of {analyte}
INTRODUCTION
The following evaluation guidelines were developed to provide chemists of the Methods
Development Team with a uniform and practical means for evaluating sampling and analytical
methods that utilize chromatographic techniques. The guidelines define sampling and
analytical parameters, specify required laboratory tests, statistical calculations, and
criteria for acceptance, and provide a detailed outline for the written reports. An
overview of the guidelines is shown in Figure 1 The overall goal of
these guidelines is to provide OSHA with sampling and analytical methods that can be
clearly defended with evaluation data.
These guidelines are continually open to examination by the OSHA Methods Development
Team who are using them, and refinements are formally made on a periodic basis. The
resulting evolution in the guidelines is apparent when comparing early methods to more
recent ones. The evaluation guidelines have been effectively used and refined for more than
twenty years. Revisions in this September 1999 update include the addition of evaluation
tests for diffusive samplers.
Active sampling is defined as collection of an analyte using a sampling pump to draw air
through an appropriate adsorbent. Diffusive sampling is a passive technique which collects
the analyte without a sampling pump using the principles of diffusion.
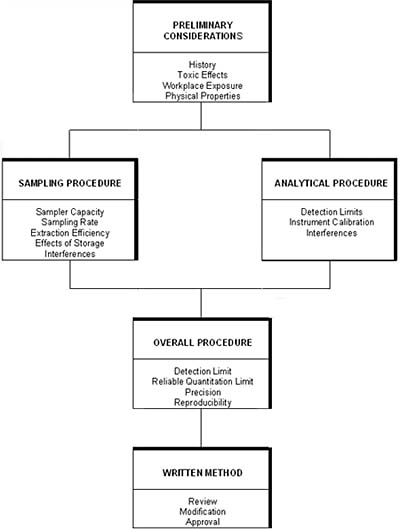
Figure 1. Evaluation scheme for OSHA chromatography methods. |
EVALUATION GUIDELINES
- Preliminary Considerations
- Review literature and consult appropriate sources for information on the following:
Existing or related sampling and analytical procedures
Toxic effects
Workplace exposure (what industries and how many people involved)
Physical properties and other descriptive information
Potential interferences
- Determine the analyte concentration at which the evaluation will be performed. This
value, which shall be known as the target concentration (TC), may be an OSHA PEL, an ACGIH
TLV, or some other concentration for which there is a basis for selection.
- Consider both active and diffusive samplers for vapors. The ideal goal is to provide
sampling options for both types of samplers, if possible. Filters or OSHA Versatile
Samplers (OVS) are to be considered for collecting aerosols.
Perform initial tests to determine the following parameters of the procedure: analytical
conditions, capacity of the selected sampling device, extraction solvent, and internal
standard (if used). Carbon disulfide shall be the first choice as an extraction solvent
for adsorbent tubes and diffusive samplers analyzed by GC/FID. If this is inadequate,
consider the solvent mixtures currently in use at SLTC before formulating a new extraction
solvent (i.e., 60/40 dimethylforamide / carbon disulfide, 95/5 carbon disulfide / isopropyl
alcohol or 95/5 methylene chloride / ethanol).
- Analytical Procedure
- Detection Limit of the Analytical Procedure (DLAP)
Detection limits, in general, are defined as the amount (or concentration) of analyte
that gives a response (YDL) that is significantly
different (three standard deviations (SBR)) from the
response (YBR) of a reagent blank.
| YDL - YBR = 3SBR |
(1) |
| where |
SBR is the standard deviation of a reagent blank
YDL is the response at the detection limit
YBR is the response of the reagent blank |
|
The direct measurement of YBR and
SBR in chromatographic methods is typically inconvenient
and difficult because YBR is usually extremely low.
Estimates of these parameters can be made with data obtained from the analysis of a series
of analytical standards whose responses are in the vicinity of the response of a reagent
blank. The regression curve obtained for a plot of instrument response versus concentration
of analyte will usually be linear. If it is clearly nonlinear, refer to
Burkhart1 for alternate
calculations. Assuming SBR and the precision of data about
the curve are similar, the standard error of estimate for the regression curve can be
substituted for SBR in the above equation. The standard
error of estimate of a line is the mathematical equivalent of the standard deviation for
tabulated data. The following calculations derive a formula for the detection limit:
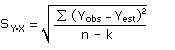 |
where |
SY·X |
is the standard error of estimate for the detection limit |
| Yobs |
is observed response |
| Yest |
is estimated response from regression curve |
| n | is total number of data points |
| k | is 2 for a linear regression |
At point YDL on the regression curve
| YDL = A(LD) + YBR |
| where |
YDL is the response at the detection limit (slope)
LD is the detection limit
A is analytical sensitivity
YBR is the response of the background |
|
therefore
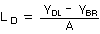
Substituting for YDL from Equation 1 gives
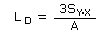 (2)
(2)
- Use the following procedure to assure that the concentrations of analytical
standards used to determine the regression curve will produce responses in the vicinity of
the background response:
- Estimate the background response near the elution time of the analyte from a reagent blank.
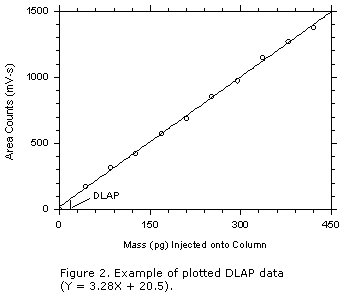 Prepare ten standards, in equally spaced intervals, with the highest standard
producing a signal about ten times the background response.
Prepare ten standards, in equally spaced intervals, with the highest standard
producing a signal about ten times the background response.
- Analyze the ten analytical standards and one reagent blank.
- Determine the regression line and the standard estimate of error from the data by
plotting response versus mass injected onto the column.
- Calculate the DLAP using Equation 2. Report the DLAP in the method as mass of analyte
injected onto the head of the column.
- Prepare a graph of the DLAP data as shown in Figure 2 for inclusion in the method.
- The detection limit of the overall procedure (DLOP) and the reliable quantitation
limit (RQL), described in Sections
IV.A and
IV.B, can be determined in conjunction with this test.
Instrument Calibration
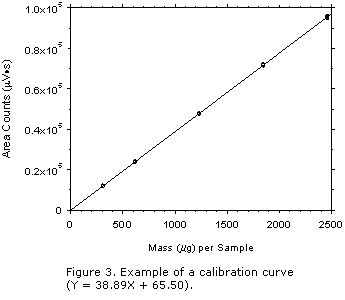 Report the standard error of estimate from the linear regression of data points over
a range that covers 0.25 to 2 times the target concentration with the highest mass loading
at the recommended sampling time for each sampler tested. The data for the line is
determined from the triplicate analysis of analytical standards at the following
concentrations: 0.25, 0.5, 1, 1.5, and 2 times the target concentration. The standard error
of estimate measures the variation or scatter about the line of
regression.2
Report the standard error of estimate from the linear regression of data points over
a range that covers 0.25 to 2 times the target concentration with the highest mass loading
at the recommended sampling time for each sampler tested. The data for the line is
determined from the triplicate analysis of analytical standards at the following
concentrations: 0.25, 0.5, 1, 1.5, and 2 times the target concentration. The standard error
of estimate measures the variation or scatter about the line of
regression.2
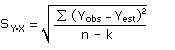
| where |
SY·X |
is the standard error of estimate |
| Yobs |
is observed response |
| Yest |
is estimated response from regression curve |
| n |
is total number of data points |
| k |
is 2 for a linear regression |
- Prepare two stock standards from the same NIST-traceable (if possible) standard.
Dilute each stock to the required five (5) concentrations. Inject each diluted standard
three times.
- Use the data collected to construct the calibration curve for inclusion in the
method, as shown in
Figure 3 (Section 3.5.2)
- Generate a chromatogram of a standard at the target concentration for inclusion in
the method. (Section 3.5.1)
- Interferences to the Analytical Procedure
- Interferences to the analytical method make identification and quantitation of the
analyte difficult or impossible.
- Determine the effects of suspected interferences by analyzing spiked analytical
standards. Avoid serious interferences to the analytical method by modifying the method or
collection procedure.
- If a reagent has been added to the sampling media, generate a chromatogram (for
inclusion in the method) of a sample at the target concentration showing the extra peak's
relationship to the analyte. (Section 3.5.1)
- Qualitative Analysis
Present a mass spectrum or alternate chromatographic conditions that will aid in
confirming the identity or purity of the analyte (or derivative) peak. Mass spectrometry
may provide the most conclusive identification and should be addressed in all cases, even
if this amounts to an explanation why it is not possible or not available. Peak response
ratios and analysis with alternate detectors may also be useful. Use the format of
Section 3.5.1 to present analytical conditions with chromatograms,
UV spectra, or mass spectra. Include this information in the method. (Section 4.10)
- Sampling Procedure
These evaluation guidelines address the evaluation of samplers containing adsorbent
media or filters and may require slight modification for the adequate evaluation of more
unique samplers such as those utilizing reactive reagents, or those containing both
adsorbent and filter components. Modification may also be required for the evaluation of
bubbler sampling procedures. Consider bubblers only as a sampling technique of last resort.
Specific requirements which apply to the evaluation of diffusive samplers are included in
the appropriate sections.
Active Samplers - Sampling Rate and Capacity
- For those substances that have a peak, ceiling, or short-term exposure limit,
determine the limitations of taking a short-term sample (applicable time from
Table Z-2 or expanded health standards of 29 CFR 1910) at the selected sampling
rate. If a short-term sample collected at the recommended sampling rate does
not result in a mass of analyte equal to or greater than 10 times the RQL, study the use of
a higher flow rate through additional breakthrough studies. For ceiling exposure limits
listed in Table Z-1, determine if 15 minutes is practical as the recommended
sampling time.
- Select a sampling rate that is suitable for the active sampler. The goal is to have
a 4-hour recommended sampling time for TWA samples. {Use 50-200
mL/min for tubes and 1-2 L/min for filters and OSHA Versatile Samplers OVS).}
- Sampler capacity is defined by the length of time a sampler {front adsorbent section
only for two-section tubes} can be used under a set of known test conditions
without significant loss of analyte. It can also be described as a corresponding air volume
or as a collected analyte mass. Use breakthrough tests to determine sampler capacity.
Consider breakthrough to have occurred when the effluent from the active sampler contains a
concentration of analyte that is 5% of the upstream concentration (5% breakthrough). This
can be determined by monitoring the downstream effluent with an instrument such as a total
hydrocarbon analyzer, a gas chromatograph, or an infrared spectrophotometer, after the
response of the upstream concentration has been established. When instrumental monitoring
of the downstream effluent is not possible, monitor breakthrough with a backup sampler that
is changed at measured time intervals and analyzed. Determine the analyte concentration in
the effluent, at the midpoint of each time interval, from the air volume sampled in each interval.
- Determine breakthrough at ambient temperature from a test atmosphere containing an
analyte concentration equal to 2 times the target concentration. Use an absolute humidity
for the test atmosphere of 15.7 milligrams of water per liter of air (about 80% relative
humidity at 22.2°C). All test atmospheres generated throughout these guidelines must
be non-condensing.
- Repeat breakthrough tests to assure reproducibility. {Three tests total.}
- Prepare a plot of breakthrough data for inclusion in the method as shown in Figure 4.
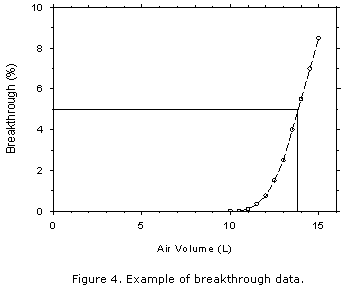
- Select whichever is shorter, a recommended sampling time
of 4 h or 80% of the time required to exceed the capacity of the sampler when challenged at
two times the target concentration.
- Retention Efficiency
Retention efficiency is the percentage of analyte retained on a spiked sampler after a
predetermined volume of appropriately conditioned air is drawn through it.
Test for retention of the analyte by using one set of six samplers to sample a test
atmosphere containing two times the target concentration at an absolute humidity of 15.7
milligrams of water per liter of air (about 80% relative humidity at 22.2°C) for
one-quarter of the recommended sampling time. Discontinue sampling and set
three samplers aside. Flush the generation system with contaminant-free air.
Resume sampling with three samplers from contaminant-free humid air for
three-quarters of the recommended sampling time. Analyze the six samplers. The
test fails if the mean of the recoveries of the second half is 90% or less of the mean
recovery of the first three samples. If the test passes, the recommended sampling time
is the value from Paragraph
III.A.7
If the first test fails, repeat the test by using another set of six samplers to sample
the same test atmosphere but reduce times by one-half. If the test passes, the new
recommended sampling time is one-half of the old value. If the mean of the
recoveries of the second half of the set is 90% or less of the mean recovery of the first
three samples, consider retention inadequate and an alternate sampling procedure must be
considered.
If an atmosphere can not be generated, retention efficiency may be tested in the following manner:
For adsorbent tubes, spike the sampler in a manner that places the analyte at the head
of the adsorbent bed. One way of accomplishing this, if the analyte is volatile, is to
place the analyte on the glass wool plug immediately ahead of the adsorbent tube. The
analyte will be rapidly leached to the head of the adsorbent bed when the test is started.
If liquid injection of the analyte onto the adsorbent bed must be used, care should be
taken to assure it is injected onto the head of the adsorbent bed. Retention efficiency
tests are useful when it is not possible to perform breakthrough tests with controlled test
atmospheres. They will provide partial support of a sampler capacity by showing that
analyte present on the sampler can be retained when the recommended sampling conditions are
used.
- Spike six samplers with an amount of analyte equivalent to the two times the target
concentration based on a tentative recommended air volume.
- Select a recommended sampling time that is suitable for the samplers and draw air
through them for 1.25 times the recommended sampling time.
- The absolute humidity of the air drawn through the samplers shall be approximately
15.7 milligrams of water per liter of air (about 80% relative humidity at 22.2°C).
- Retention efficiency is determined by analyzing (including extraction or extraction
efficiency corrections) the spiked samplers after air has been drawn through them. During
the test, the downstream effluent shall be monitored as it would in a breakthrough test.
- Filters and support pads (if used) are extracted separately and the extractant of each is analyzed
to determine the retention efficiency. If support pads are used, spike six filters as in Step 'a' and
place in separate sealed cassettes, with backup pads, for 4 h with no air pulled through them.
These filters will be used as controls to determine if contamination of the support pad occurs
before air is pulled through the cassette.
- Test for the effect of low humidity on collection efficiency by using a set of three samplers to sample
a test atmosphere containing two times the target concentration at an absolute humidity of 3.9
milligrams of water per liter of air (about 20% relative humidity at 22.2°C) or less using the
recommended sampling time. Upon analysis, all three front sections of the individual samples
should have collected enough analyte to be greater than 90% of the theoretical amount. If not, an
alternate sampling procedure must be considered.
- Test for the effect of low concentration on collection efficiency by using a set of three samplers to
sample a test atmosphere containing 0.1 times the target concentration at an absolute humidity of
15.7 milligrams of water per liter of air (about 80% relative humidity at 22.2°C) for the
recommended sampling time. Upon analysis, all three front sections of the individual samples
should have collected enough analyte to be greater than 90% of the theoretical amount. If not, an
alternate sampling procedure must be considered.
- Test for the effect of at least one suspected interference on collection efficiency by using a set of
three samplers to sample for the recommended sampling time a test atmosphere at an absolute
humidity of 15.7 milligrams of water per liter of air (about 80% relative humidity at 22.2°C)
containing the target concentration, and the suspected interference at a concentration set to an
appropriate level. The appropriate level for the interference will be its PEL or TLV. If more than one
interference is used, then the concentration of the interference will be divided by the number of
interferences used. If two interferences are used, each will have a concentration equal to one-half
of its PEL or TLV. Upon analysis, all three samples should have each collected greater than 90%
of the theoretical amount of the analyte. If 10% or more of the analyte is found on the back section,
the recommended sampling time may be too long. Repeat the breakthrough test (Steps 3-8) with
the interferences present in the atmosphere to determine a shorter recommended sampling time.
- Diffusive Samplers - Sampling Rate and Capacity
{It is necessary to generate a controlled test atmosphere to determine sampling rates and capacities
for diffusive samplers. Before making these determinations, the preliminary extraction efficiency from
wet absorbent should be determined. Calculate the mass of analyte that will be collected on the
diffusive sampler for four hours from an atmosphere containing the target concentration using an
approximate sampling rate based on the manufacturer's literature (e.g., SKC is 13 mL/min or 3M is 31
mL/min). Spike at least two samplers with this amount of analyte and another two samplers with 5%
of the amount. Upon analysis, the values should be ±10% of each other. Use the average as the
preliminary extraction efficiency. After the preliminary sampling rate and preliminary recommended
sampling time are determined with the preliminary extraction efficiency, perform the final extraction
efficiency studies in Section
III.C Using the final extraction efficiency,
recalculate the final sampling rate and final recommended sampling time.}
- For those substances that have a peak, ceiling, or short-term exposure limit, determine the
limitations of taking a short-term sample (applicable time from Table Z-2 or expanded health
standards of 29 CFR
1910). The shortest
recommended sampling time for a short-term sample should result in a mass of analyte equal
to or greater than 10 times the RQL. For ceiling exposure limits listed in Table Z-1,
determine if 15 minutes is practical as the recommended sampling time.
- Determine sampling rates using replicate samples collected at increasing time intervals from a
controlled test atmosphere. Collect three samples for each time interval. The time intervals will
normally be 5, 10, and 30 min plus 1, 2, 3, 4, 6, 8, and 10 hours. The concentration of the test
atmosphere should be two times the target concentration. If the analyte is in Table Z-2, use two
times the TWA PEL. The absolute humidity of 15.7 milligrams of water per liter of air (about 80%
relative humidity at 22.2°C) should be used. The concentration of the test atmosphere should be
verified with an alternate method. Two alternate methods are needed if the first alternate method
and the theoretical concentration do not agree. (alternate methods may include an active sampling
procedure and online monitoring with instruments such as GC or IR.) The face velocity of the test
atmosphere over the samplers should be approximately 0.4 m/s. Record the temperature and
pressure inside the sampling chamber. The masses are corrected for extraction efficiency, as
determined in Section
III.C. Analytical data from only the primary sorbent
section of samplers that have a secondary sorbent section should be used in these tests. Sampling
rate is expressed in milliliters per minute, and will be calculated by the following equation:
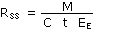
| where |
RSS |
is sampling rate at sampling site |
| M | is mass collected |
| C | is concentration of the test atmosphere |
| t | is sampling time |
| EE | is extraction efficiency |
- Convert the ambient sampling rates, which are determined at ambient temperature and atmospheric
pressure, to equivalent sampling rates at the NTP conditions of 760 mmHg and 298 K with the
following equation:3
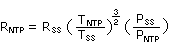
| where |
RNTP |
is the sampling rate at NTP conditions |
| RSS | is the sampling rate at sampling site |
| TSS | is the temperature in K |
| TNTP | is 298.2 K |
| PSS | is the pressure at the sampling site |
| PNTP | is 760 mmHg |
- Plot the sampling rates against sampling times as shown in the following example. Find the
preliminary sampling rate by averaging the nine values for the 0.5, 1 and 2-h samples {12.2
mL/min}. Draw horizontal lines that are 10% above and below the preliminary sampling rate {13.42
and 10.98 mL/min}. Average all of the sampling rates from 5 min through 10 h that are between the
lines to determine the sampling rate. This range should contain at least four of the time intervals
and the relative standard deviation of the sampling rate should be no more than 5%. {Report the
mean (12.1 mL/min), standard deviation (0.445 mL/min) and the relative standard deviation (3.7%)
for all of the data points used to determine the sampling rate.} Report the sampling rate as milliliters
per minute at 101.3 kPa and 25°C and the range of time it covers, for example, 5 min to 4 h.
Table 1
Determination of Samping Rate
and Recommended Sampling Rate
|
| time (h) |
sampling rate (mL/min)
|
| first |
second |
third |
|
5 min
10 min
0.5
1
2
3
4
6
8
10 |
12.4
12.3
12.1
12.0
12.1
12.0
11.8
11.4
11.2
10.2 |
12.5
12.4
12.2
12.2
12.2
12.1
11.9
11.5
11.0
10.3 |
12.6
12.5
12.3
12.3
12.4
12.2
12.0
11.6
11.1
10.1 |
|
|
|
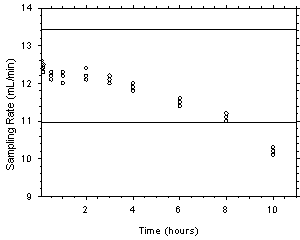
Figure 5. Example of plotted data to determine the recommended sampling time and sampling rate.
|
-
-
- To determine the recommended sampling time, use the data from the previous paragraph. Sampler
capacity is defined to be exceeded when the sampling rate appears to decrease rapidly. Find the
data point with the longest time that is between the horizontal lines. Multiply this time by 0.80 to
determine the maximum sampling time {6.4 h}. If this time is over 4 h, the recommended sampling
time is 4 h. This will provide a conservative safety margin when samples are taken in complex
work atmospheres where substances may compete for sites on the adsorbent. Report the sampler
capacity as mass of analyte collected on the sampler if it is allowed to sample an atmosphere
containing two times the target concentration for the recommended sampling time.
- Test for reverse diffusion of the analyte by using one set of six samplers to sample a test
atmosphere containing two times the target concentration at an absolute humidity of 15.7 milligrams
of water per liter of air (about 80% relative humidity at 22.2°C) for one-quarter
of the recommended sampling time. Discontinue sampling and set three samplers aside. Flush the
generation system with contaminant-free air. Resume sampling with the other three
samplers from contaminant-free humid air for three-quarters of the
recommended sampling time. Analyze the six samplers. The test fails if the mean of the recovered
masses of the second half is 90% or less of the mean of first three samples. If the test passes,
the recommended sampling time is the value from the previous paragraph.
If the first test fails, repeat the test by using another set of six samplers to sample the same test
atmosphere but reduce all time by one-half. If the test passes, the new recommended sampling
time is one-half of the old value. If the mean of the recovered masses of the second half of the set
is 90% or less of the mean of the first three samples, consider reverse diffusion significant and an
alternate sampling procedure must be considered.
- Test for the effect of low humidity on sampler performance by exposing a set of three samplers to
a test atmosphere containing two times the target concentration at an absolute humidity of 3.9
milligrams of water per liter of air (about 20% relative humidity at 22.2°C) or less for the
recommended sampling time. Upon analysis, all three of the individual samples should have
collected enough mass to be greater than 90% of the theoretical amount. If not, an alternate
sampling procedure must be considered. Use sampling rate to calculate the theoretical amount.
- Test for the effect of low concentration on sampler performance by exposing a set of three samplers
to a test atmosphere containing 0.1 times the target concentration at an absolute humidity of 15.7
milligrams of water per liter of air (about 80% relative humidity at 22.2°C) for the recommended
sampling time. Upon analysis, each of the three individual samples should have collected enough
mass to be greater than 90% of the theoretical amount. If not, an alternate sampling procedure
must be considered. Use sampling rate to calculate the theoretical amount.
- Test for the effect of at least one suspected interference on sampler performance by using a set of
three samplers to sample for the recommended sampling time a test atmosphere containing the
target concentration at an absolute humidity of 15.7 milligrams of water per liter of air (about 80%
relative humidity at 22.2°C), and the suspected interference at a concentration set at an appropriate
level. The appropriate level for the interference will be its PEL or TLV. If more than one
interference is used, then the concentration of the interference will be divided by the number of
interferences used. If two interferences are used, each will have a concentration equal to one-half
of its PEL or TLV. Upon analysis, all three of the individual samples should have collected enough
mass to be greater than 90% of the theoretical amount. If not, repeat the breakthrough test (steps
1-4) with the interferences present in the atmosphere to determine a shorter recommended
sampling time. Use sampling rate to calculate the theoretical amount.
- Extraction Efficiency
- First determine the minimum amount of time required to extract a constant amount from a sample.
A series of spiked samplers are to be extracted and analyzed while increasing the amount of time
between extraction and analysis. Shake each sample by hand for a few seconds shortly after
adding the solvent. If the time exceeds 1 h, determine if mechanical agitation can reduce the time
to fully extract the sample.
- Perform a test of the extraction efficiency with wet samplers. Pull an air volume equivalent to the
recommended sampling time through four active samplers and expose four diffusive samplers for
the recommended sampling time using a contaminant-free atmosphere containing an absolute
humidity of 15.7 milligrams of water per liter of air (about 80% relative humidity at 22.2°C) or spike
each sampler with 50 µL of water. Spike the wet active and diffusive samplers at one times the
target concentration. {If there are several target concentrations, select the target concentration and
recommended sampling time combination which will produce the highest mass loading on the
sampler.} If there is a significant difference in the mean of the wet sampler's extraction recovery
from the mean dry sampler's extraction recovery, repeat the test. A significant difference is when
the mean of the wet samplers is more than two standard deviations from the mean of the dry
sampler at the same mass loading. If the difference persists, change the sampler or extraction
solvent to minimize the difference.
- The extraction efficiency is the mean percent of analyte recovered from dry samplers and
determined at the RQL, and 0.25, 0.5, 1, 1.5, and 2 times the target concentration, based on the
recommended air volume. A dry sampler is one that is used as received from the manufacturer.
The average of all six determinations will be the extraction efficiency for the analytical procedure
if they are similar. In the event the extraction efficiency does not remain constant at lower sample
loadings, a plot of extraction efficiency versus concentration should be constructed and included
in the method.
- Prepare four samplers and three standards at each of the six concentrations.
- Store the spiked samples at room temperature for a sufficient time to assure complete adsorption
of the analyte. although the time required may vary with each particular analyte, the samples
should be stored overnight unless a shorter time period can be justified.
- Extract the spiked samples. After an appropriate amount of time for equilibrium to occur, analyze
the samples. Reseal two of the dry samples containing the target concentration amount of analyte
immediately after analysis for use in the test described in Step 9. Prepare the analytical standards
with the same microliter syringe used in spiking the extraction samples. Compare the samples to
the respective standards to determine the percent recovered.
- Calculate the extraction efficiency as follows:
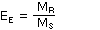
| where |
EE is extraction efficiency
MR is mass recovered
MS is mass spiked |
- An average extraction efficiency >75% is acceptable but >90% is perferred.
- Determine the stability of extracted dry samples by reanalyzing the four dry target concentration
extraction samples one day after the extraction efficiency was determined. Reseal two of the four
vials containing these samples with new septa after the initial analysis. The remaining two samples
shall retain their punctured septa. Use freshly prepared standards in the reanalysis. The results
obtained from the resealed samples will determine if restrictions must be placed on how soon after
extraction the samples must be analyzed. The results from the samples stored with punctured
septa will determine if restrictions must be placed on the reanalysis of samples that may sit (as in
autosampler trays) for a period of time before reanalysis. Consider extracted samples stable if the
difference between the extraction efficiency one day after extraction and the extraction efficiency
from the initial determinations is not greater than 10% for each sample. Also determine the number
of punctures in each septum during the injection of the sample and report this number.
- If storage instability is detected in Step 9, a time study may be necessary in which extracted
samples are reanalyzed at sufficiently short time intervals. Use this data to determine how long
after extraction (or analysis) a valid analysis (or reanalysis) can be performed. Use the criteria for
sample stability in Step 9.
- If support pads are used in conjunction with filters, determine their extraction efficiency by spiking
them with a sample loading equivalent to 0.05 times the target concentration.
- Effects of Storage
- Collect thirty-three samples from a controlled test atmosphere containing the analyte at the target
concentration. The absolute humidity should be 15.7 milligrams of water per liter of air (about 80%
relative humidity at 22.2°C). Use the recommended sampling time and sampling rate NTP. If sample
collection is extremely time consuming, increase the test atmosphere concentration or increase the
sampling rate in order to obtain the correct analyte loading on the samplers within a reasonable
time. If this approach is taken, make certain that sampler capacity is not exceeded due to the
altered sampling conditions.
- Analyze three samples on the day they are collected.
- Store fifteen samples at room temperature in the dark, and store the remaining 15
samples under refrigeration at a temperature of 2-6°C.
- Analyze three samples from each set approximately every third day so that the
storage test is at least 15 days in length.
- Measure recovery from the regression curve obtained by plotting percent recovery
(not corrected for extraction efficiency) versus days of storage.
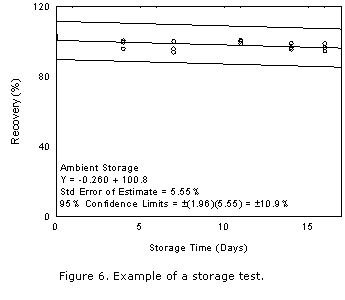 A change in recovery of more than 10% in 15 days is a significant uncorrectable bias
and must be avoided. Also, the recovery (not corrected for extraction efficiency) must
remain above 75% during storage. When these conditions are not met, they may be
overcome by use of: an alternate sampling medium, refrigerated storage requirements,
or time requirements for completion of the analysis. The preferable goal is the use a
convenient sampler without restrictions on storage conditions, or time requirements for
completion of analysis. The effectiveness of ambient shipment to the laboratory and
then storing the samples in a refrigerator until analysis can be estimated. This is
done by tracking cumulative sample loss on the plot for the ambient storage test for the
first five days and then switching to the plot for the reduced temperature test for the
remainder of the storage time.
A change in recovery of more than 10% in 15 days is a significant uncorrectable bias
and must be avoided. Also, the recovery (not corrected for extraction efficiency) must
remain above 75% during storage. When these conditions are not met, they may be
overcome by use of: an alternate sampling medium, refrigerated storage requirements,
or time requirements for completion of the analysis. The preferable goal is the use a
convenient sampler without restrictions on storage conditions, or time requirements for
completion of analysis. The effectiveness of ambient shipment to the laboratory and
then storing the samples in a refrigerator until analysis can be estimated. This is
done by tracking cumulative sample loss on the plot for the ambient storage test for the
first five days and then switching to the plot for the reduced temperature test for the
remainder of the storage time.
- Use alternate methods of preparing storage samples when safety considerations or
other problems prevent generation of dynamically test atmospheres. The alternate methods
include static test atmospheres, prepared in gas-sampling bags; vapor-spiked samples, volatilizing the analyte directly upstream from the
sampling tube; and liquid-spiked samples, injecting the analyte directly onto
the sampling tube. Introduce water by drawing the recommended amount of humid air through
the spiked sampling tube. In this last method, a small volume of humid air can be drawn
through the sampling tube so it has initial exposure to water before the analyte is
introduced. These alternate methods may require that the analyte be contained in a
solvent.
- Plot storage test data as shown in Figure 6. Note that this figure includes data
for the overall precision, which is defined in a following section. The scale on the
vertical axis is from 0% to 120%.
- Overall Procedure
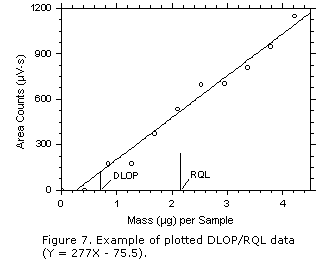 Detection Limit of the Overall Procedure (DLOP)
Detection Limit of the Overall Procedure (DLOP)
- Determine DLOP using the same procedure that was used to
determine DLAP (Section
II.A), except data shall be obtained
from spiked samplers instead of analytical standards.
- Report the DLOP as mass per sample and as an equivalent
air concentration based on the recommended sample air volume.
- Prepare a plot of the DLOP data for inclusion in the method as shown in Figure 7.
- Reliable Quantitation Limit (RQL)
- Consider the RQL as the lower limit for precise quantitative measurements.
Employing the regression line data used to calculate the DLOP, determine the RQL with the
following formula, providing the recovery from the sampler which is closest to the RQL, is
100 ± 25% of its theoretical value.
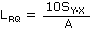
| where |
LRQ |
is the reliable quantitation limit |
| SY·X |
is the standard error of estimate for the regression line |
| A |
is the analytical sensitivity (slope) |
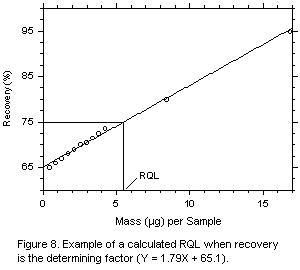 If the recovery from the closest sampler is not within 25% of its theoretical value,
then the RQL will be equal to the lowest spiked concentration that is ±25% of its
theoretical value. Determine this from a plot of recovery versus mass, as shown in Figure
8, for inclusion in the method. Additional data points are obtained by spiking a series of
samplers with 2, 3, 4, or 5 times the highest mass spiked for the DLOP.
If the recovery from the closest sampler is not within 25% of its theoretical value,
then the RQL will be equal to the lowest spiked concentration that is ±25% of its
theoretical value. Determine this from a plot of recovery versus mass, as shown in Figure
8, for inclusion in the method. Additional data points are obtained by spiking a series of
samplers with 2, 3, 4, or 5 times the highest mass spiked for the DLOP.
- Report the RQL as mass per sample and as an equivalent air concentration based on
the recommended sample air volume.
- Generate a chromatogram of the RQL for inclusion in the method.
- Determination of the Precision
- Use data from Effects of Storage (Section III.D) in the
determination of the overall precision.
- Determine the standard error of estimate for the regression
curve4,5 of each storage test with the following formula.
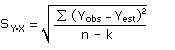
| where |
SY·X |
is the standard error of estimate |
| Yobs | is observed response |
| Yest | is estimated response from regression curve |
| n | is total number of data points |
| k | is 2 for a linear regression |
| k | is 3 for a quadratic regression |
- The standard error of estimate is determined for each sampler from the data used in both storage
tests. Use the ambient test if the restrictions are satisfied in Section III.D.6. Use the standard error
of estimate from the refrigerated storage test if the ambient test fails. If the refrigerated storage test
also fails, restrictions must be set on the maximum storage time that will be allowed before samples
must be analyzed.
- Active Sampler
Determine the total standard error of the overall procedure for each storage test (SEE) by
including the sampling pump variability (VSP) with the following formula, use an arbitrary value
of 5%.
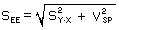
| where |
SEE |
is the overall standard error of estimate |
| SY·X |
is the standard error of estimate from storage |
| VSP |
is the sampling pump variability |
- Diffusive Sampler
Modification of the calculation for standard error of estimate is required for
diffusive samplers because VSP is not an applicable
parameter. In its place use sampling rate variability (VSR),
which is considered a function of sampler design and must be determined before methods
development work with the sampler is performed. {Because diffusive sampling rates are a
function of temperature (T) and pressure (P), the standard error of estimate
must include additional uncertainty when these parameters are not determined at the
sampling site.}
The formula for the determination of standard error of estimate for diffusive samplers
thus becomes:
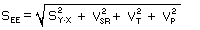
| where |
SEE |
is the overall standard error of estimate |
| SY·X |
is the standard error of estimate from storage |
| VSR |
is the variability in the sampling rate |
| VT |
is the variability in the sampling rate due to temperature |
| VP |
is the variability in the sampling rate due to pressure |
but when the sampling temperature and pressure are known, it simplifies to:
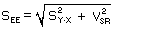
| where |
SEE |
is the overall standard error of estimate |
| SY·X |
is the standard error of estimate from storage |
| VSR |
is the variability in the sampling rate |
Determine the variability in the sampling rate from a factorial test, similar to that of the NIOSH
protocol6 or the SLTC
protocol7,8
for the validation for diffusive samplers. The variability in the sampling rate for SKC 575
Series Passive Sampler and the 3M 3520 Organic Vapor Monitor was determined to be
8.7%9 and 7.4%10,
respectively.
- Assuming a normal distribution of values about the regression curve and uniformity
of variation about the entire range of the curve, ±1.96 times the overall standard
error of estimate will represent the 95% confidence limits.
- Represent the overall precision data graphically in the method as shown in
Figure 6, and use the
overall standard error of estimate derived from the data that reflects the recommended temperature
for sample shipment to describe the method.
- The confidence limits of the overall procedure must be equal to or less than 25%.
{The rest of this section is not related to the development of a method but is included as information
that could be useful when analyzing field samples.}
- When the temperature at the sampling site is unknown, a value of 7.7% is used for VT. This is an
estimate of the maximum variability in sampling rate caused by a temperature range of 22.2 ± 15°C
(72 ± 27°F). When the sampling site temperature is known, VT is equal to zero.
- When the pressure at the sampling site is unknown, determine it from the estimated elevation of
the sampling site, and a value of 3% is used for VP. This is the
variability in pressure caused by variations due to weather, which is based on the tracking of
atmospheric pressure variations for a year at SLTC. When the pressure at the sampling site is
known, VP is equal to zero.
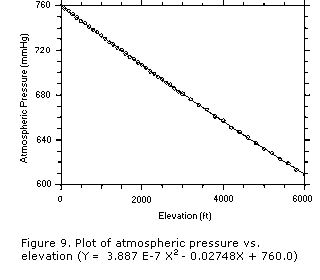 If the elevation of the sampling site is unknown, the elevation can be estimated by
data found at the World Wide Web address
AirNav.com. Select the AIRPORTS button. Select LOOK BY TOWN/REGION. Enter the city
name. Check HELIPORTS and PRIVATE. This will identify all public airports, military
airfields, private landing strips and all locations that accept helicopters. Select the
radius of the search area. Select an airfield that is close to the sampling site. Maps are
displayed to help with the selection of the nearest airfield. The elevation will be listed
near the top of the airfield's information. Use the equation in Figure 9 to estimate the
atmospheric pressure of the sampling site.
If the elevation of the sampling site is unknown, the elevation can be estimated by
data found at the World Wide Web address
AirNav.com. Select the AIRPORTS button. Select LOOK BY TOWN/REGION. Enter the city
name. Check HELIPORTS and PRIVATE. This will identify all public airports, military
airfields, private landing strips and all locations that accept helicopters. Select the
radius of the search area. Select an airfield that is close to the sampling site. Maps are
displayed to help with the selection of the nearest airfield. The elevation will be listed
near the top of the airfield's information. Use the equation in Figure 9 to estimate the
atmospheric pressure of the sampling site.
Table 211
Atmospheric Pressure Versus Elevation |
elevation
(ft) |
pressure
(mmHg) |
elevation
(ft) |
pressure
(mmHg) |
elevation
(ft) |
pressure
(mmHg) |
elevation
(ft) |
pressure
(mmHg) |
elevation
(ft) |
pressure
(mmHg) |
0
100
200
300
400
500
600
700
800
900 |
760
757
755
752
749
746
744
741
738
736 |
1000
1100
1200
1300
1400
1500
1600
1700
1800 |
733
730
727
725
722
720
717
714
712 |
1900
2000
2100
2200
2300
2400
2500
2600
2700 |
709
707
704
701
699
696
694
691
689 |
2800
2900
3000
3200
3400
3600
3800
4000
4200 |
686
683
681
676
671
667
661
657
651 |
4400
4600
4800
5000
5200
5400
5600
5800
6000 |
647
642
637
632
628
623
619
613
609 |
-
- Reproducibility
- Prepare six samples (for each target concentration and each sampler) in the same
manner as storage samples. Submit them to SLTC for analysis. Include a draft copy of the
analytical procedure for analyst instructions. Relying on the draft copy for instruction,
the chemist will analyze the samples. If the samples are stored before analysis, the
conditions under which they are stored should correspond to the recommended storage
conditions of the method. If the analyte has a ceiling, peak or STEL, generate another set
of reproducibility samples if the mass of analyte for the short-term sample
is less than 10% of the mass collected for a long-term sample.
- No individual analytical result should deviate from the theoretical value by more
than 1.96 times the standard error of estimate. If this does occur, steps must be taken to
determine and eliminate the cause of the excessive imprecision (e.g., an unanticipated
technical problem or a lack of clarity in the analytical instructions provided in the
draft copy). The reproducibility test must then be repeated.
PREPARATION OF WRITTEN REPORTS
Written reports fall into three basic categories:
-
Evaluated Methods - Sampling and analytical methodology that has been
thoroughly evaluated according to the evaluation guidelines.
- Partially Evaluated Methods - Sampling and analytical procedures for
which an in-depth evaluation has not been performed. The evaluation of these
methods is often performed rapidly in order to meet the immediate need of field personnel
when established methodology does not exist.
- Studies - Investigations that involve a class or group of analytes,
or an aspect of methodology that may be common to many methods in general. Unsuccessful
evaluations will be reported as studies.
Prepare each type of report in accordance with the following respective formats:
- Evaluated Methods
The following format provides a means of reporting data obtained during evaluation of
chromatographic sampling and analytical methods. The cover page is intended as a quick
reference that provides basic information. The backup data section contains tabulated and
graphical laboratory data that are referenced throughout the report. This outline was
prepared from the viewpoint of a chromatographic analysis.
All evaluated methods completed by the Methods Development Team will have the following
statement on the cover page:
"Evaluated method. This method has been subjected to the established evaluation
procedures of the Methods Development Team."
Page Numbering - Do not number the cover page. Number pages at the bottom, including
the method number followed by a dash and then the page number. Example: The first page
after the cover page of Method 1001 would be "1001-1".
Comments are set off with braces "{ }", and are not included in the method.
Text written in 10 point Arial font with full justification with no hyphenation
Tabs: Cover page - 2.0 - Method - 0.2, 0.59, 1.12, 1.36
OSHA logo on cover page - size = 0.500", paragraph anchor, 0" horizontal, 0" from top,
right margin, wrap behind text
Tables - 9 point Arial font, 0.02" for left inside margin, right inside margin, top
row margin, bottom row margin
Graphs - size = 3.1", paragraph anchor, 0" horizontal, 0" from top, right margin, wrap
left, caption is 9 point Arial font
Table boxes - size = 3.1, paragraph anchor, 0" horizontal, 0" from top, left margin if
next to a graph, wrap left or neither, 9 point Arial font
References will follow as closely as possible the format recommended by the American
Chemical Society in their 1997 edition of "The ACS Style Guide - A Manual for Authors and Editors."
{ANALYTE}
{as listed in CFR or ACGIH}
| Method number: |
1xxx |
| |
Target concentration:
OSHA PEL:
ACGIH TLV: |
____ ppm (____ mg/m3)
____ ppm (____ mg/m3) {None if no PEL}
____ ppm (____ mg/m3) {None if no TLV} |
| |
| Procedure: |
Active samples are collected by drawing workplace air through ____ {active
sampler} with personal sampling pumps. Diffusive samples are collected
by exposing ____ {diffusive sampler} to workplace air. Samples are
extracted with ____ and analyzed by ____ using a ____ detector. |
| |
Recommended sampling time and sampling rate:
{Active sampler}:
{Diffusive sampler}: |
____ min at ____ mL/min (____ L)
{If the sampling rate is over 250 mL/min, use L/min.}
____ min |
| |
Reliable quantitation limit:
{Active sampler}:
{Diffusive sampler}: |
____ ppm (____ mg/m3)
____ ppm (____ mg/m3) |
| |
Standard error of estimate at the target concentration:
{Active sampler}:
{Diffusive sampler}: |
____%
____%*
*For samples where sampling site atmospheric pressure and temperature
are known. When either or both of these values are unknown, see Section
4.4 for applicable standard errors of estimate. |
| |
| Special requirements: |
When using a {diffusive sampler}, report the sampling site pressure and
temperature. {If none, delete this item} |
| |
| Status of method: |
Evaluated method. This method has been subjected to the established
evaluation procedures of the Methods Development Team. |
| |
| ____ {month year} |
{Chemist} ____ |
Methods Development Team
Industrial Hygiene Chemistry Division
OSHA Salt Lake Technical Center
Salt Lake City UT 84115-1802
-
1. General Discussion
{The backup data section will be referenced throughout the method in the following
manner: "(Section 4.____)". Literature citations will be footnotes.}
- 1.1 Background
-
1.1.1 History
{Explain why past methodology is inadequate, and how the new procedure is superior.
Also, obvious questions that may be raised by knowledgeable readers should be
addressed. Keep length to 1.5 pages or less.}
1.1.2 Toxic effects (This section is for information only and should not
be taken as the basis of OSHA policy.)
{Cite sources for presented information. If both animal data and human data are presented,
present the animal data first. If the entire section is taken from one reference, the
reference notation can be placed behind the qualifying statement in the heading.}
1.1.3 Workplace exposure
{Report major sources of exposure in the workplace and, if available, the size of the work
population that is exposed. If the entire section is taken from one reference, the reference
notation can be placed behind the heading.}
1.1.4 Physical properties and descriptive information {These are to be used if applicable,
others properties may be listed.}
| CAS number: |
____ |
vapor pressure:{kPa (mmHg)} |
____ |
| IMIS number: |
____ |
l max: |
____ |
| molecular weight: |
____ |
flash point: |
____ |
| boiling point: |
____ |
odor: |
____ |
| melting point: |
____ |
lower explosive limit: |
____ |
| appearance: |
____ |
synonyms: |
____ |
| specific gravity: |
____ |
structural formula: |
____ |
| molecular formula: |
____ |
solubility: |
____ |
GUIDE This method was evaluated according to the OSHA SLTC "EVALUATIONLINES FOR AIR SAMPLING
METHODS UTILIZING CHROhods/cMATOGRAPHIC ANALYSIS".12 The Guidelines define analytical parameters,
specify required laborhe anaatory tests, statistical calculations and acceptance criteria. Tlyte air
concentrations throughical pout this method are based on the recommended sampling and analytarameters.
Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg).
-
1.2 Limit defining parameters
- 1.2.1 Detection limit of the analytical procedure
The detection limit of the analytical procedure is ____ {mass}. This is the amount of
analyte that will give a detector response that is significantly different from the response
of a reagent blank. (Section 4.1) {If the definition for the analytical
detection limit for a particular analyte must be altered, the altered definition should appear
in this section and the detailed explanation should appear in Section 4.1.}
1.2.2 Detection limit of the overall procedure
The detection limits of the overall procedure are ____ {mass} per sample (____ ppm or
____ mg/m3) and ____ {mass} per sample (____ ppm or ____ mg/m3) for {active
sampler} and {diffusive sampler}, respectively. These are the amounts of {analyte} spiked
on the respective sampler that will give detector responses that are significantly different
from the responses of respective sampler blanks. (Sections 4.2)
1.2.3 Reliable quantitation limit
The reliable quantitation limits are ____ {mass} per sample (____ ppm or ____ mg/m3) and
____ {mass} per sample (____ ppm or ____ mg/m3) for {active sampler} and {diffusive
sampler}, respectively. These are the amounts of {analyte} spiked on the respective
samplers that will give detector responses that are considered the lower limits for precise
quantitative measurements. (Section 4.2)
1.2.4 Instrument calibration
{Active sampler}
The standard error of estimate is ____ {mass} over the range of ____ to ____ µg. This
range corresponds to 0.25 to 2 times the target concentration. (Section 4.3)
{Diffusive sampler}
The standard error of estimate is ____ {mass} over the range of ____ to ____ µg. This
range corresponds to 0.25 to 2 times the target concentration. (Section 4.3)
1.2.5 Precision
{Active sampler}
The precision of the overall procedure at the 95% confidence level for the ambient
temperature {or reduced temperature ( ____°C)} 15-day storage test (at the target
concentration) from {adsorbent tube} is ± ____ %. This includes an additional 5% for
sampling pump variability. (Section 4.4) {The precision cited must be based on the storage
data that reflects the temperature recommended for shipment of samples.}
{Diffusive sampler}
Table 1.2.5
Precision of the Overall Procedure
|
| known conditions |
precision (±%) |
|
both T & P
only T
only P
neither T nor P |
____
____
____
____ |
|
The precisions of the overall procedure at the 95% confidence level for the ambient
temperature {or reduced temperature ( ____°C)} 15-day storage test (at
the target concentration) from {diffusive sampler} are given in Table 1.2.5. They each
include an additional ____% for sampling rate variability. There are different values
given, depending on whether both, either, or neither temperature (T) or
atmospheric pressure (P) are known at the sampling site. If the sampling site
temperature is unknown, it is assumed to be 22.2 ± 15°C (72 ± 27°F)
and a variability of ±7.7% is included. If the atmospheric pressure is not known, it
is estimated from the sampling site elevation and a variability of ±3% is included.
(Section 4.4) {The precision cited must be based on the storage
data that reflects the temperature recommended for shipment of samples.}
1.2.6 Recovery
The recovery of {analyte} from samples used in a ____-day storage test remained above
____ % and ____ % {the lowest points on the regression curves of
Section 4.5.} when the
samples were stored at ____ °C for {active sampler} and {diffusive sampler}, respectively.
(or if the case requires: The recovery of {analyte} from samples used in a ____-day storage
test remained above 75% for the first ____ days when samples were stored at ____ °C.)
(Section 4.5)
1.2.7 Reproducibility
Six samples for both samplers collected from a controlled test atmosphere {or spiked by
liquid injection, etc.} were submitted for analysis by the OSHA Salt Lake Technical Center.
The samples were analyzed according to a draft copy of this procedure after ____ days of
storage at ____ C. No individual sample result deviated from its theoretical value by
more than the precision reported in Section 1.2.5. (Section 4.6)
-
2. Sampling Procedure
All safety practices that apply to the work area being sampled should be followed. The sampling
equipment should be attached to the worker in such a manner that it will not interfere with work
performance or safety.
-
2.1 Apparatus {Provide general descriptions of the required equipment followed by a description of specific equipment actually used in the evaluation, if applicable.}
2.1.1 {Active sampler}
Example:
Samples are collected with {description of the sampler, 7-cm × 4-mm i.d. ×
6-mm o.d. glass sampling tubes packed with two sections of {adsorbent}}.
{The front section contains 110 mg and the back section contains 55 mg of {adsorbent}.
{The sections are held in place with glass wool plugs.} For this evaluation,
commercially prepared {active samplers} were purchased from {Supplier}, Inc. (catalog no.
____).
Samples are collected using a personal sampling pump calibrated, with the sampling
device attached, to within ±5% of the recommended flow rate.
2.1.2 {Diffusive sampler}
Samples are collected with a {diffusive sampler}. For this evaluation, commercially
available samplers were purchased from {Supplier}, Inc. (catalog no. xxx-xx).
A thermometer and barometer to determine the sampling site air temperature and
atmospheric pressure.
- 2.2 Reagents
{If no reagents are required, state "None required". Otherwise use the format described
in Section 3.2.}
-
2.3 Technique {Describe steps involved in sample collection, preparation, and shipment.}
- 2.3.1 {Adsorbent tube}
Immediately before sampling, break off the ends of the flame-sealed tube as to provide an
opening approximately half the internal diameter of the tube. Wear eye protection when
breaking ends. Use tube holders to minimize the hazard of broken glass. All tubes should
be from the same lot.
The smaller section of the adsorbent tube is used as a back-up and is positioned nearest
the sampling pump. Attach the tube holder to the sampling pump so that the adsorbent
tube is in an approximately vertical position with the inlet facing down during sampling.
Position the sampling pump, tube holder and tubing so they do not impede work
performance or safety.
Draw the air to be sampled directly into the inlet of the tube holder. The air being sampled
is not to be passed through any hose or tubing before entering the sampling tube.
After sampling for the appropriate time, remove the adsorbent tube and seal it with plastic
end caps. Seal each sample end-to-end with an OSHA-21 form as
soon as possible.
Submit at least one blank sample with each set of samples. Handle the blank sampler in
the same manner as the other samples except draw no air through it.
Record sample air volume (liters), sampling time (minutes) and sampling rate (mL/min) for
each sample, along with any potential interferences on the OSHA-91A form.
Submit the samples to the laboratory for analysis as soon as possible after sampling. If
delay is unavoidable, store the samples at refrigerator temperature. Ship any bulk samples
separate from the air samples.
2.3.2 SKC 575-002 Samplers (In general, follow the manufacturer's instructions.)
Remove the sampler enclosed in an air-tight clear bag from the container. Keep the
O-ring, press-on cover, cover retainer, port plugs and PTFE
tube for later use.
Remove the sampler from the clear bag when ready to begin sampling. CAUTION - The
monitor immediately begins to sample when it is removed from this bag.
Record the start time on the sampler label or on the Form OSHA-91A.
Attach the sampler to the worker near his/her breathing zone with the perforations in the
sampler facing out. Assure that the area directly in front of the sampler is unobstructed
throughout the sampling period.
At the end of the sampling period, immediately detach the sampler from the worker and
attach the cover with the O-ring in place onto the sampler using the cover retainer.
Visually inspect the O-ring to be sure it is forming a proper seal around the entire
circumference of the sampler. Record the stop time on sampler label or on OSHA-91A
form.
Prepare a blank by removing an unused sampler from its clear package and immediately
attaching a cover with the O-ring in place onto it.
Seal each sampler with an OSHA-21 form.
Verify that the sampling times are properly recorded on the OSHA-91A form for each
sample. Also, identify blank samples on this form.
Record the room temperature and atmospheric pressure of the sampling site on the Form
OSHA-91A.
List any compounds that could be considered potential interferences, especially solvents,
that are being used in the sampling area.
Submit the samples to the laboratory for analysis as soon as possible after sampling. If
delay is unavoidable, store the samples at refrigerator temperature. Ship any bulk samples
separate from the air samples. Include all port plugs and PTFE tubes which will be used
in the laboratory analyses.
Ship any bulk sample(s) in a container separate from the air samples.
2.3.3 {Filter cassette}
Remove the plastic end plugs from the filter cassette immediately before sampling.
{Remove the rear plastic plug and the top piece of the filter cassette for open-face
sampling.}
Attach the cassette to the sampling pump so that it is in an approximately vertical position
with the inlet facing down during sampling. Position the sampling pump, cassette and
tubing so it does not impede work performance or safety.
Draw the air to be sampled directly into the inlet of the cassette. The air being sampled is
not to be passed through any hose or tubing before entering the cassette.
After sampling for the appropriate time, remove the sample and seal the cassette with
plastic end plugs {plug and top piece}. Seal each sample end-to-end with an
OSHA-21 form as soon as possible.
Submit at least one blank sample with each set of samples. Handle the blank sampler in
the same manner as the other samples except draw no air through it.
Record sample air volumes (liters) for each sample, along with any potential interferences.
Submit the samples to the laboratory for analysis as soon as possible after sampling. If
delay is unavoidable, store the samples at refrigerator temperature. Ship any bulk samples
separate from the air samples.
2.3.4 3M OVMs (In general, follow the manufacture's instructions supplied with the samplers.)
The monitors come individually sealed in small metal cans. When ready to begin
sampling, remove the plastic lid from the can and lift up on the revealed ring. Pull back on
the ring to open the can. Discard the metal top of the can and remove the monitor.
CAUTION - The monitor immediately begins to sample when the can is unsealed.
Keep the two closure caps with attached port plugs, cup and PTFE tubes in the can for
later use. Close the can with the plastic lid.
Record the start time on the back of the monitor or on the OSHA-91A form.
Attach the monitor to the worker near his/her breathing zone with the white face forward.
Assure that the area directly in front of the sampler is unobstructed throughout the
sampling period. Do not remove the white film and ring from the monitor until the sampling
period is terminated.
At the end of the sampling period, detach the monitor from the worker and remove the
white film and retaining ring. Immediately snap a closure cap onto the primary (top) section
of the monitor (where the white film and ring were removed). It is critical that this step be
done as quickly as possible because the sampling rate is more than five times faster
without the white film in place, which can be an important consideration, especially for
short-term sampling. Assure that the attached port plugs are placed firmly into the port
holes. The white film and ring can be discarded. Record the stop time on the back of the
monitor or on the OSHA-91A form.
The following steps should be performed in a low background area for a set of monitors as
soon as possible after sampling.
Ready a blank by removing the white film and ring and attaching a closure cap onto an
unused monitor.
For each monitor (one at a time), separate the primary (top) and secondary (bottom)
sections of the monitor using the edge of a coin as a pry.
Securely snap a cup onto the bottom of the primary section.
Snap a closure cap onto the secondary section of the monitor and assure that the attached
port plugs are placed firmly into the port holes.
Return the sampler sections with closure caps and cup in place to the metal can which
contains the PTFE tubes (which will be used by the laboratory). Close the can with the
plastic lid, and seal it with an OSHA-21 form.
Verify that the sampling times are properly recorded on OSHA-91A form for each sample.
Also, identify blank samples on this form.
Record the room temperature and atmospheric pressure of the sampling site on
OSHA-91A form.
List any compounds that could be considered potential interferences, especially solvents,
that are being used in the sampling area.
Submit the samples to the laboratory for analysis as soon as possible after sampling. If
delay is unavoidable, store the samples at refrigerator temperature. Ship any bulk samples
separate from the air samples.
- 2.4 Sampler capacity (Section 4.7) {Describe test, conditions and results.}
- 2.4.1 The sampling capacity of the front section of an {adsorbent} sampling tube was tested by
sampling a dynamically generated test atmosphere of {analyte} (____ mg/m3 or ____ ppm)
at an absolute humidity of 15.7 milligrams of water per liter of air (about 80% relative
humidity at 22.2°C). The samples were collected at ____ mL/min. The 5% breakthrough
sampling time was determined to be ____ min.
2.4.2 The sampling rate and capacity of the {diffusive sampler} were determined by sampling a
dynamically generated test atmosphere of {analyte} (____ mg/m3 or ____ ppm) at an
absolute humidity of 15.7 milligrams of water per liter of air (about 80% relative humidity
at 22.2°C) for increasing time intervals. A sampling rate of ____ mL/min and sampling
time of ____ min were obtained from this test.
- 2.5 Extraction efficiency (Section 4.8)
It is the responsibility of each analytical laboratory to determine the extraction efficiency because
the adsorbent material, internal standard, reagents and laboratory techniques may be different than
the those listed in this evaluation and influence the results.
2.5.1 {Active sampler}
The mean extraction efficiency for {analyte} from dry {adsorbent} over the range of {RQL
or 0.05} to 2 times the target concentration (____ to ____ milligrams per sample) was
____%. The extraction efficiency was not affected by the presence of water. {A significant
difference is when the mean of the wet samplers is more than two standard deviations from
the mean of the dry sampler at the same mass loading.}
Extracted samples remain stable for at least ____ h {or days}.
2.5.2 {Diffusive sampler}
The mean extraction efficiency for {analyte} from dry {diffusive sampler} over the range of
{RQL or 0.05} to 2 times the target concentration (____ to ____ milligrams per sample) was
____%. The extraction efficiency was not affected by the presence of water. {A significant
difference is when the mean of the wet samplers is more than two standard deviations from
the mean of the dry sampler at the same mass loading.}
Extracted samples remain stable for at least ____ h {or days}.
2.6 Recommended sampling time and sampling rate
2.6.1 {Active sampler}
Sample for up to ____ min at ____ mL/min (____ L) when using {active sampler} to collect
TWA (long-term) samples.
Sample for ____ min at ____ mL/min (____ L) when using {active sampler} to collect
ceiling (short-term) samples.
When short-term samples are collected, the air concentration equivalent to the reliable
quantitation limit becomes larger. For example, the reliable quantitation limit for {active
sampler} is ____ ppm (____ mg/m3) for {analyte} when ____ L are collected.
2.6.2 {Diffusive sampler}
Sample for up to ____ min when using {diffusive sampler} to collect TWA (long-term)
samples. The sampling rate is ____ mL/min.
Sample for ____ min when using {diffusive sampler} to collect ceiling (short-term)
samples. The sampling rate is ____ mL/min.
When short-term samples are collected, the air concentration equivalent to the reliable
quantitation limit becomes larger. For example, the reliable quantitation limit for {diffusive
sampler} is ____ ppm (____ mg/m3) for {analyte} when ____ L are collected.
- 2.7 Interferences, sampling (Section 4.9)
2.7.1 {Active sampler}
The retention efficiency for all samples was above ____% {report the lowest value}, when
{active samplers} containing ____ mg {½× TC} of {analyte} were allowed to sample ____
L of contaminant-free air having an absolute humidity of 15.7 milligrams of water per liter
of air (about 80% relative humidity at 22.2°C).
The collection efficiency for all samples was above ____% of theoretical {report the lowest
value}, when {active samplers} were used to sample a test atmosphere containing two
times the target concentration of {analyte} and having an absolute humidity of 3.9
milligrams of water per liter of air (about 20% relative humidity at 22.2°C).
The collection efficiency for all samples was above ____% of theoretical {report the lowest
value}, when {active samplers} were used to sample a test atmosphere containing 0.1
times the target concentration of {analyte} and having an absolute humidity of 15.7
milligrams of water per liter of air (about 80% relative humidity at 22.2°C).
The collection efficiency for all samples was above ____% of theoretical {report the lowest
value}, when {active samplers} were used to sample a test atmosphere containing one
times the target concentration of {analyte}, ____ mg/m3 of {interference} and having an
absolute humidity of 15.7 milligrams of water per liter of air (about 80% relative humidity
at 22.2°C).
2.7.2 {Diffusive sampler}
The mass for all samples was above ____% {report the lowest value}, when {diffusive
samplers} containing ____ mg {½× TC} of {analyte} were used to sample for
three-quarters of the recommended sampling time contaminant-free
air having an absolute humidity of 15.7 milligrams of water per liter of air (about
80% relative humidity at 22.2°C).
The recovery for all samples was above ____% of theoretical {report the lowest value},
when {diffusive samplers} were used to sample a test atmosphere containing two times
the target concentration of {analyte} and having an absolute humidity of 3.9 milligrams of
water per liter of air (about 20% relative humidity at 22.2°C).
The recovery for all samples was above ____% of theoretical {report the lowest value},
when {diffusive samplers} were used to sample a test atmosphere containing 0.1 times the
target concentration of {analyte} and having an absolute humidity of 15.7 milligrams of
water per liter of air (about 80% relative humidity at 22.2°C).
The recovery for all sample was above ____% of theoretical {report the lowest value},
when {diffusive samplers} were used to sample a test atmosphere containing one times
the target concentration of {analyte}, ____ mg/m3 of {interference} and having an absolute
humidity of 15.7 milligrams of water per liter of air (about 80% relative humidity at 22.2°C).
3. Analytical Procedure
Adhere to the rules set down in your Chemical Hygiene Plan.13
Avoid skin contact and inhalation of all chemicals and review all MSDSs.
3.1 Apparatus {Provide general descriptions of the required equipment. Follow each general
description with a specific description of equipment actually used in the evaluation.}
Example:
- 3.1.1 Gas chromatograph equipped with an FID. A Hewlett-Packard Model 6890 was used in this evaluation.
3.2 Reagents {Provide general descriptions of the required reagents. Follow each general description
with a description of the specific reagent actually used in the evaluation.}
Example:
-
3.2.1 Methylene chloride, [CAS no.], ___ grade or better. The methylene chloride used in this evaluation was A.C.S. HPLC grade (lot no. Q87C654) purchased from Aldrich (Milwaukee, WI).
3.2.2 Carbon disulfide (CS2), [CAS no.], ____ grade or better. The carbon disulfide used in this
evaluation was low benzene grade (lot no. 37529) purchased from JT Baker Chemical Co.
(Phillipsburg, NJ).
3.3 Standard preparation {Describe preparation of standards in general and give an example.}
Example:
- 3.3.1 Prepare concentrated stock standards of {analyte} in CS2. Prepare working analytical
standards by injecting microliter amounts of concentrated stock standards into 2-mL vials
containing 1 mL of extracting solution delivered from the same dispenser used to extract
samples. For example, to prepare a target level standard, inject ____ L of a stock
solution containing ____ mg/mL of {analyte} in CS2 into 1 mL of extracting solution.
3.3.2 Bracket sample concentrations with standard concentrations. If upon analysis, sample
concentrations fall outside the range of prepared standards, prepare and analyze additional
standards to confirm instrument response, or dilute high samples with extraction solvent
and reanalyze the diluted samples.
3.4 Sample preparation {Describe steps involved in preparing samples for analysis.}
Example:
- 3.4.1 {Active sampler}
Remove the plastic end caps from the sample tube and carefully transfer each section of
the adsorbent to separate 2-mL vials. Discard the glass tube and glass wool plugs.
Add 1.0 mL of extracting solution to each vial and immediately seal the vials with
polytetrafluoroethylene-lined caps.
Shake the vials vigorously several times during the ____ min extraction time.
3.4.2 SKC 575-002 Samplers (In general, follow the manufacturer's instructions.)
Cut off the ends of the two protruding tubes of each sampler with a razor blade or sharp
knife.
Slowly add 1.0 mL of extraction solvent through one of the protruding tubes (ports). After
about 30 seconds, slowly add another 1.0 mL of extraction solvent.
Immediately insert plugs into the ports.
Mount the samplers in the sampler rack (SKC Cat. No. 226-04-5) of a specialized shaker
(SKC Cat. No. 226D-03-1) and shake the samplers for 1 hour.
Do not leave the extracted sample in the sampler. Transfer each extracted sample by
removing the plugs from the sampler ports, firmly inserting the tapered end of a supplied
PTFE tube into the outer port and carefully pouring the solution through the PTFE tube into
a labeled autosampler vial.
3.4.3 3M 3520 OVMs (In general, follow the manufacturer's instructions.)
Remove both sampler sections from the metal cans, along with the sections of PTFE
tubing. Assure that the closure caps are firmly snapped to the primary and secondary
sections of all the samplers. Also assure that all cap plugs are firmly seated in the cap
ports. Any deviations must be noted.
Prepare one section of sampler at time by temporarily removing the cap plugs from the
ports and adding 2.0 mL of extraction solvent through the center port. This is most easily
done by dispensing two 1.0-mL aliquots of extraction solvent using a dispenser.
Immediately replace the plugs in the ports.
Allow the sampler sections to extract for 30 min. Periodically apply gentle agitation to the
sampler sections during the extraction period.
Transfer the solution from each sampler section by removing both plugs from the ports,
inserting a decanting spout (a small section of PTFE tubing) into the rim port and pouring
the liquid through the spout into a labeled autosampler vial. Immediately cap each vial.
- 3.5 Analysis
Example:
-
3.5.1 Analytical conditions {Provide detailed instrument settings, include chromatogram
at the target concentration, and the calibration technique used.}
Example:
GC conditions
|
|
|
column
temperature: |
60°C (column) |
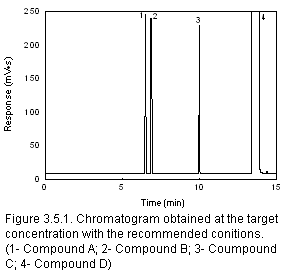 |
zone
temperatures: |
250°C (injector)
300°C (detector) |
| run time: |
15 min |
| column gas flow: |
1.2 mL/min (hydrogen) |
| septum purge: |
1.5 mL/min (hydrogen) |
| injection size: |
1.0 µL
(12.5:1 split) |
| column: |
60-m × 0.32-mm i.d. capillary SPB-5 (df = 1.0-µm) |
| retention times: |
6.5 min (compound A)
6.9 min (compound B)
10.0 min (compound C)
13.6 min (compound D) |
| |
FID conditions
|
| hydrogen flow: |
34 mL/min |
| air flow: |
450 mL/min |
| nitrogen makeup flow: |
33 mL/min |
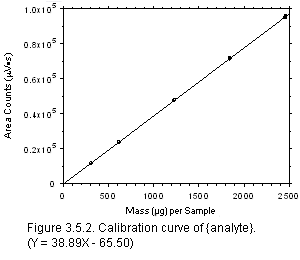
3.5.2 An internal standard (ISTD) calibration method is used. A calibration curve can
be constructed by plotting ISTD-corrected response of standard injections
versus micrograms of analyte per sample. Bracket the samples with freshly prepared
analytical standards over a range of concentrations.
-
3.6 Interferences (analytical)
Example:
3.6.1 Any compound that produces an FID response and has a similar retention time as
the analyte or internal standard is a potential interference. If any potential
interferences were reported, they should be considered before samples are extracted.
Generally, chromatographic conditions can be altered to separate an interference from
the analyte.
3.6.2 When necessary, the identity of an analyte peak may be confirmed with additional
analytical data (Section 4.9).
-
3.7 Calculations {Use 24.46 L/mol [(22.41 L/mol)(298.2 K)/273.2 K] for the molar volume.}
Example:
3.7.1 {Active sampler)
The amount of {analyte} per sampler is obtained from the appropriate calibration curve in
terms of micrograms per sample, uncorrected for extraction efficiency. The back section
is analyzed primarily to determine the extent of sampler saturation. If any analyte is found
on the back section, it is added to the amount on the front section. This total amount is
then corrected by subtracting the total amount (if any) found on the blank. The air
concentration is calculated using the following formulas.
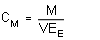
| where |
CM |
is concentration by weight (mg/m3) |
| M | is micrograms per sample |
| V | is liters of air sampled |
| EE |
is extraction efficiency, in decimal form |
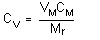
| where |
CV | is concentration by volume (ppm) |
| VM | is molar volume at 25°C |
| CM | is concentration by weight |
| Mr | is molecular weight |
3.7.2 {Diffusive sampler]
The amount of {analyte} for the samples is obtained from the appropriate calibration curve
in terms of micrograms per sample, uncorrected for extraction efficiency. {The back section
is analyzed primarily to determine the extent of sampler saturation. If any analyte is found
on the back section, the amount is multiplied by 2.2 (as per manufacturer's instructions)
and then added to the amount on the front section.} This {total} amount is then corrected
by subtracting the {total} amount (if any) found on the blank. The air concentration is
calculated using the following formulas.

| where |
RSS |
is the sampling rate at sampling site |
| RNTP | is the sampling rate at NTP conditions |
| TSS | is the sampling site temperature in K |
| TNTP | is 298.2 K |
| PSS | is the sampling site pressure in mmHg |
| PNTP | is 760 mmHg |
3.7.2 {Diffusive sampler]
The amount of {analyte} for the samples is obtained from the appropriate calibration curve
in terms of micrograms per sample, uncorrected for extraction efficiency. {The back section
is analyzed primarily to determine the extent of sampler saturation. If any analyte is found
on the back section, the amount is multiplied by 2.2 (as per manufacturer's instructions)
and then added to the amount on the front section.} This {total} amount is then corrected
by subtracting the {total} amount (if any) found on the blank. The air concentration is
calculated using the following formulas.

| where |
RSS |
is the sampling rate at sampling site |
| RNTP | is the sampling rate at NTP conditions |
| TSS | is the sampling site temperature in K |
| TNTP | is 298.2 K |
| PSS | is the sampling site pressure in mmHg |
| PNTP | is 760 mmHg |
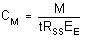
| where |
CM |
is concentration by weight (mg/m3) |
| M | is micrograms per sample
|
| RSS | is the sampling rate at the sampling site |
| t | is the sampling time |
| EE | is extraction efficiency, in decimal form |
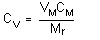
| where |
CV |
is concentration by
volume (ppm) |
| VM |
is molar volume at
25 °C |
|
| CM |
is concentration by
weight |
|
| Mr |
is molecular weight |
|
If the sampling site temperature is not provided, assume that it is 22.2°C. If
the sampling site atmospheric pressure is not given, calculate an approximate value
based on the sampling site elevation from the following equation.
| PSS = AE2 - BE + 760.0 |
where |
PSS |
is the approximate atmospheric pressure |
| E |
is the sampling site elevation, ft |
| A |
is 3.887×10-7 mmHg/ft2 |
| B |
is 0.02748 mmHg/ft |
- 4. Backup Data {This section contains evaluation data which is referenced in the preceding sections.}
General background information about the determination of detection limits and precision of the overall
procedure is found in the "Evaluation Guidelines for Air Sampling Methods Utilizing Chromatography
Analysis".14 The Guidelines define analytical
parameters, specific laboratory tests, statistical calculations and acceptance criteria.
-
4.1 Detection limit of the analytical procedure (DLAP) {Present the test data in a table and a graph.}
Example:
The DLAP is measured as the mass of analyte introduced onto the chromatographic column. Ten
analytical standards were prepared with equal increments with the highest standard containing ____
µg/mL. This is the concentration that would produce a peak approximately 10 times the response
of a reagent blank near the elution time of the analyte. These standards, and the reagent blank
were analyzed with the recommended analytical parameters (1-µL injection with a __:1 split), and
the data obtained were used to determine the required parameters (standard error of estimate and
slope) for the calculation of the DLAP. Values of ____ and ____ were obtained for the slope and
standard error of estimate respectively. DLAP was calculated to be ____ pg.
Table 4.1
Detection Limit of the Analytical Procedure
|
concentration
(µg/mL) |
mass on column
(pg) |
area counts
(µV-s) |
|
0
0.421
0.841
1.26
1.68
2.10
2.52
2.94
3.36
3.78
4.21 |
0
42.1
84.1
126
168
210
252
294
336
378
421 |
0
173
318
425
573
690
853
973
1150
1270
1380 |
|
|
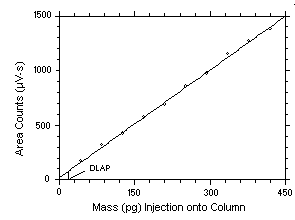
Figure 4.1. Plot of data to determine the DLAP.
(Y = 3.28X + 20.5)
|
4.2 Detection limit of the overall procedure (DLOP) and reliable quantitation limit
(RQL) {Present the test data in a table, graph and a chromatogram of the RQL.}
Example:
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based
on the recommended sampling parameters. Ten samplers were spiked with equal descending
increments of analyte, such that the highest sampler loading was ____ µg/sample. This is the
amount spiked on a sampler that would produce a peak approximately 10 times the response of a
sample blank. These spiked samplers, and the sample blank were analyzed with the recommended
analytical parameters, and the data obtained used to calculate the required parameters (standard
error of estimate and the slope) for the calculation of the DLOP. Values of ____ and ____ were
obtained for the slope and standard error of estimate respectively. The DLOP was calculated to be
____ µg/sample (____ ppm, ____ mg/m3).
Table 4.2
Detection Limit of the Overall Procedure
|
mass per sample
(µg) |
area counts
(µV-s) |
|
0
0.421
0.841
1.26
1.68
2.10
2.52
2.94
3.36
3.78
4.21 |
0
0
178
177
375
536
696
703
810
948
1150 |
|
|
|
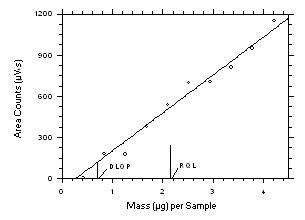
Figure 4.2.1. Plot of data to determine the DLOP/RQL. (Y = 277X - 75.8)
|
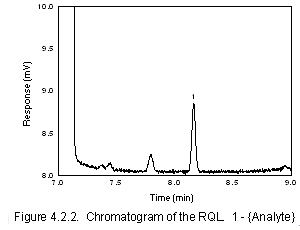 The RQL is considered the lower limit for precise quantitative measurements. It is
determined from the regression line parameters obtained for the calculation of the DLOP,
providing 75% to 125% of the analyte is recovered. The RQL is ____ µg per sample
(____ ppm, ____ µg/m3) Recovery at this concentration
is ____%.
The RQL is considered the lower limit for precise quantitative measurements. It is
determined from the regression line parameters obtained for the calculation of the DLOP,
providing 75% to 125% of the analyte is recovered. The RQL is ____ µg per sample
(____ ppm, ____ µg/m3) Recovery at this concentration
is ____%.
4.3 Instrument calibration
Example:
The standard error of estimate was determined from the linear regression of data
points from standards over a range that covers 0.25 to 2 times the target concentration
for the sampler with the highest mass loading. A calibration curve was constructed and
shown in Section 3.5.2 from the six injections of five standards. The standard error of
estimate is ____ {mass}.
Table 4.3.
Instrument Calibration
|
standard concn
(µg/mL) |
area counts
(µV • s) |
|
307
615
1230
1845
2461 |
11935
23988
47783
71790
95054 |
11795
23738
47593
71490
95987 |
11888
23741
47895
71901
95616 |
11735
23938
47793
71795
95087 |
11988
23747
47595
71495
95916 |
11895
23788
47883
71990
95654 |
|
4.4 Precision (overall procedure)
- 4.4.1 {Active sampler}
The precision at the 95% confidence level is obtained by multiplying the standard error
of estimate by 1.96 (the z-statistic from the standard normal distribution at
the 95% confidence level). In
Section 4.5, 95% confidence intervals are drawn about their
respective regression lines in the storage graph figures. The precision of the overall
procedure of ±____ % was obtained from the standard error of estimate of ____ in
Figure ____. {The standard estimate of error listed on the cover page of the method must
be based on the storage data that reflects the temperature recommended for shipment and
storage of samples.}
4.4.2 {Diffusive sampler}
Table 4.4.2
Standard Error of Estimate
and Precision of the Overall Procedure
|
| known conditions |
precision (±%) |
|
both T & P
only T
only P
neither T nor P |
____
____
____
____ |
|
The precisions of the overall procedure at the 95% confidence level for the ambient temperature {or reduced temperature ( ____ °C)} 15-day storage test (at the target concentration) from {diffusive sampler} are given in Table 4.4.2. They each include an additional ____% for sampling rate variability. There are different values given, depending on whether both, either, or neither temperature (T) or atmospheric pressure (P) are known at the sampling site. If the sampling site temperature is unknown, it is assumed to be 22.2 ± 15°C (72 ± 27°F) and a variability of ±7.7% is included. If the atmospheric pressure is not known, it is estimated from the sampling site elevation and a variability of ±3% is included. {The standard error of estimate listed on the cover page of the method must be based on the storage data that reflects the temperature recommended for shipment and storage of samples.}
4.5 Storage test {Describe the storage test, including preparation of samples.}
- 4.5.1 {Active sampler}
Storage samples for {analyte} were prepared by collecting samples from a controlled test atmosphere using the recommended sampling conditions. The concentration of {analyte} was at the target concentration with an absolute humidity of 15.7 milligrams of water per liter of air (about 80% at 22.2°C). Thirty-three storage samples were prepared. Three samples were analyzed on the day of generation. Fifteen of the tubes was stored at reduced temperature (4°C) and the other fifteen was stored in a closed drawer at ambient temperature (about 22°C). At 2-5 day intervals {preferably 3-day intervals}, three samples were selected from each of the two storage sets and analyzed. Sample results are
not corrected for extraction efficiency.
Table 4.5.1
Storage Test for {Analyte}
|
time
(days) |
ambient storage
recovery (%) |
|
refrigerated storage
recovery (%) |
|
0
4
7
11
14
16 |
103.5
99.6
100.0
100.8
95.6
96.5 |
101.6
100.5
95.8
98.8
96.6
94.5 |
101.9
95.8
93.8
100.2
99.1
98.8 |
|
103.5
99.3
95.9
100.6
98.9
99.3 |
101.6
99.4
100.9
103.6
99.6
99.5 |
101.9
101.8
95.8
105.5
97.5
99.1 |
|
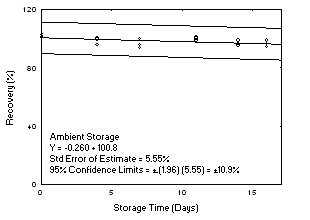
Figure 4.5.1.1. Ambient storage test for {analyte}. |
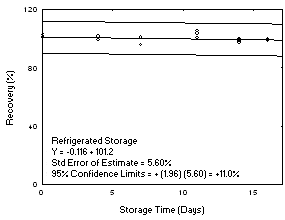
Figure 4.5.1.2. Refrigerated storage test for {analyte}. |
4.5.2 {Diffusive sampler}
Storage samples for {analyte} were prepared by collecting samples from a controlled test atmosphere using the recommended sampling conditions. The concentration of {analyte} was at the target concentration and the test atmosphere with an absolute humidity of 15.7 milligrams of water per liter of air (about 80% at 22.2°C). Thirty-three storage samples were prepared. Three samples were analyzed on the day of generation. Fifteen of the tubes was stored at reduced temperature (4°C) and the other fifteen was stored in a closed drawer at ambient temperature (about 22°C). At 2-5 day intervals {preferably 3-day intervals}, three samples were selected from each of the two storage sets and analyzed. Sample results are
not corrected for extraction efficiency.
Table 4.5.2
Storage Test for {Analyte}
|
time
(days) |
ambient storage
recovery (%) |
|
refrigerated storage
recovery (%) |
|
0
4
7
11
14
16 |
103.5
99.6
100.0
100.8
95.6
96.5 |
101.6
100.5
95.8
98.8
96.6
94.5 |
101.9
95.8
93.8
100.2
99.1
98.8 |
|
103.5
99.3
95.9
100.6
98.9
99.3 |
101.6
99.4
100.9
103.6
99.6
99.5 |
101.9
101.8
95.8
105.5
97.5
99.1 |
|

Figure 4.5.2.1. Ambient storage test for {analyte}. |

Figure 4.5.2.2. Refrigerated storage test for {analyte}. |
4.6 Reproducibility {Describe reproducibility test and present data in Tables 4.6.1
and 4.6.2. Specify that the "amount found" is corrected for extraction efficiency.}
Example:
Six samples were prepared for both types of sampler by collecting them from a
controlled test atmosphere similar to that which was used in the collection of the
storage samples. The samples were submitted to the OSHA Salt Lake Technical Center for
analysis. The samples were analyzed after being stored for ____ days at ____°C.
Sample results were corrected for extraction efficiency. No sample result for {analyte}
had a deviation greater than the precision of the overall procedure determined in
Section 4.4.
Table 4.6.1
Reproducibility Data for
{Analyte} on {Active Sampler}
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
420.6
420.6
420.6
420.6
420.6
420.6 |
388.6
395.5
393.2
379.6
379.0
406.1 |
92.4
94.0
93.5
90.3
90.1
96.6 |
-7.6
-6.0
-6.5
-9.7
-9.9
-3.4 |
|
| |
Table 4.6.2
Reproducibility Data for
{Analyte} on {Diffusive Sampler}
|
theoretical
(µg/sample) |
recovered
(µg/sample) |
recovery
(%) |
deviation
(%) |
|
420.6
420.6
420.6
420.6
420.6
420.6 |
388.6
395.5
393.2
379.6
379.0
406.1 |
92.4
94.0
93.5
90.3
90.1
96.6 |
-7.6
-6.0
-6.5
-9.7
-9.9
-3.4 |
|
|
4.7 Sampler capacity {Describe breakthrough or other studies used.}
4.7.1 {Active sampler}
The sampling capacity of the front section of an {active sampler} was tested by sampling
from a dynamically generated test atmosphere of {analyte} (____ mg/m3 or ___ ppm) with
an absolute humidity of 15.7 milligrams of water per liter of air (about 80% relative
humidity at 22.2°C). The samples were collected at ____ mL/min. A GC equipped with
a gas sampling valve and an FID was placed in-line behind the front test section and
monitored the downstream air flow every 5 min. The recommended sampling time is ____h.
Table 4.7.1
Breakthrough of {Analyte} with {Active Sampler}
|
test
no. |
air vol
(L) |
sampling
time
(min) |
downstream
concn
(mg/m3) |
break-
through
(%) |
|
| 1 |
14.2
15.8
18.7
21.5
23.0
24.5 |
285
315
375
430
460
490 |
0.00
0.00
0.72
1.96
2.91
3.94 |
0.0
0.0
1.02
2.78
4.13
5.59 |
| |
| 2 |
13.8
16.0
19.3
22.0
23.3
24.8 |
275
320
385
440
465
495 |
0.00
0.31
0.84
2.04
3.15
4.11 |
0.0
0.44
1.19
2.90
4.47
5.84 |
| |
| 3 |
13.5
15.5
18.5
21.8
22.5
25.0 |
270
310
370
435
450
500 |
0.00
0.21
0.75
1.85
3.20
4.20 |
0.0
0.30
1.07
2.63
4.55
5.97 |
|
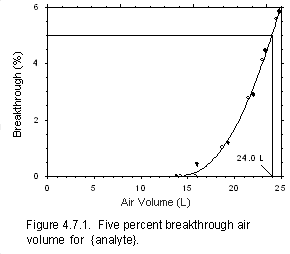
4.7.2 {Diffusive sampler}
The sampling rate and sampler capacity are determined with samples collected at
increasing time intervals from a controlled test atmosphere. Sampler capacity is exceeded
when the sampling rate decreases. The concentration of the test atmosphere was two
times the target concentration with an absolute humidity of 15.7 milligrams of water per liter
of air (about 80% at 22.2°C). The preliminary sampling rate was determined by averaging
the nine values for the 0.5, 1 and 2 h samples. Horizontal lines were placed 10% above
and below the preliminary sampling rate. The sampling rate is ____ mL/min at 760 mmHg
and 25°C and represents the average of all values between the lines. The standard
deviation and RSD are ____ mL/min and ____%, respectively. The data obtained are
shown in Table 4.7.2 and Figure 4.7.2. Mass collected is corrected for extraction
efficiency. The recommended sampling time is ____ h.
Table 4.7.2
Determination of Sampling Rate and Time
|
| time (h) |
sampling rate (mL/min)
|
| first | second | third |
|
0.083
0.167
0.5
1
2
3
4
6
8
10 |
12.4
12.3
12.1
12.0
12.1
12.0
11.8
11.4
10.9
10.6 |
12.5
12.4
12.2
12.2
12.2
12.1
11.9
11.5
11.0
10.7 |
12.6
12.5
12.3
12.3
12.4
12.2
12.0
11.6
11.1
10.5 |
|
|
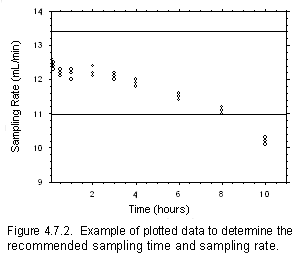
|
-
4.8 Extraction efficiency and stability of extracted samples
The extraction efficiency is dependent on the extraction solvent as well as the internal standard.
Other extraction solvents or internal standards may be used provided that the new extraction
solution or internal standard is tested. The new extraction solvent or internal standard should be
tested as described below.
-
4.8.1 {Active sampler}
Extraction efficiency
The extraction efficiencies of {analyte} were determined by liquid-spiking {active sampler}
with the analyte at the RQL to 2 times the target concentration. These samples were
stored overnight at ambient temperature and then analyzed. The mean extraction
efficiency over the working range of the RQL to 2 times the target concentration is ____%.
The extraction efficiency for the wet samplers was not included in the overall mean
because it would bias the results.
-
Table 4.8.1.1
Extraction Efficiency of {Analyte} from {Active Sampler}
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0 (wet) |
0.21
105
210
421
632
841
421 |
99.8
103.5
101.6
101.9
105.8
95.8
102.8 |
97.5
99.5
102.4
101.8
105.0
92.8
103.7 |
99.9
99.6
100.5
95.8
100.4
97.7
101.1 |
101.2
100.0
95.8
100.2
94.2
97.7
100.4 |
99.6
100.6
100.1
99.9
101.4
96.0
102.0 |
|
Stability of extracted samples
The stability of extracted samples was investigated by reanalyzing the target concentration
samples 24 h after initial analysis. After the original analysis was performed two vials were
recapped with new septa while the remaining two retained their punctured septa. The
samples were reanalyzed with fresh standards. The average percent change was ____%
for samples that were resealed with new septa and ____% for those that retained their
punctured septa. Each septum was punctured ____ times for each injection.
Table 4.8.1.2
Stability of Extracted Samples for {Analyte}
|
punctured septa replaced
|
punctured septa retained
|
initial
(%) |
after
one day
(%) |
difference
(%) |
initial
(%) |
after
one day
(%) |
difference
(%) |
|
92.8
97.3
95.1 |
89.1
88.3
(mean)
88.7 |
-3.7
-9.0
-6.4 |
98.5
97.7
98.1 |
88.8
94.6
(mean)
91.7 |
-9.7
-3.1
-6.4 |
|
- 4.8.2 {Diffusive sampler}
Extraction efficiency
The extraction efficiencies of {analyte} were determined by liquid-spiking {diffusive
sampler} with the analyte at the RQL to 2 times the target concentration. These samples
were stored overnight at ambient temperature and then extracted and analyzed. The
average extraction efficiency over the working range of RQL to 2 times the target
concentration was ____%. The extraction efficiency for the wet samplers was not included
in the overall mean because it would bias the results.
-
Table 4.8.1.2
Extraction Efficiency of {Analyte} from {Diffusive Sampler}
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
RQL
0.25
0.5
1.0
1.5
2.0
1.0(wet) |
0.21
42.1
84.1
421
632
841
421 |
99.8
101.6
101.9
95.8
102.8
96.8
103.5 |
97.5
102.4
101.8
92.8
103.7
99.8
99.5 |
99.9
100.5
95.8
97.7
101.1
98.7
99.6 |
101.2
95.8
100.2
97.7
100.4
98.7
100.0 |
99.6
100.1
99.9
96.0
102.1
98.5
100.7 |
|
Stability of extracted samples
The stability of extracted samples was investigated by reanalyzing the target concentration
samples 24 h after initial analysis. After the original analysis was performed two vials were
recapped with new septa while the remaining two retained their punctured septa. The
samples were reanalyzed with fresh standards. The average percent change was ____%
for samples that were resealed with new septa and ____% for those that retained their
punctured septa. Each septum was punctured ____ times for each injection.
Table 4.8.2.2
Stability of Extracted Samples for {Analyte}
|
punctured septa replaced
|
punctured septa retained
|
initial
(%) |
after
one day
(%) |
difference
(%) |
initial
(%) |
after
one day
(%) |
difference
(%) |
|
92.8
95.8
94.3 |
89.1
92.3
(mean)
90.7 |
-3.7
-3.5
-3.6 |
99.5
97.7
98.6 |
96.8
88.7
(mean)
92.8 |
-2.7
-9.0
-5.9 |
|
-
4.9 Interferences (sampling)
-
4.9.1 {Active sampler}
Retention
Table 4.9.1
Retention Efficiency (%) of {Analyte}
from {Active Sampler}
|
| set | 1 | 2 | 3 | mean |
|
first
second
second/first |
99.6
100.4
100.8 |
98.2
100.1
101.9 |
100.0
100.2
100.2 |
99.3
100.2
100.9 |
|
The ability of a {active sampler} to retain {analyte} after it has been collected was
tested by sampling an atmosphere containing ____ mg/m3 {two
times the target concentration} of {analyte} at an absolute humidity of 15.7 milligrams
of water per liter of air (about 80% relative humidity at 22.2°C). Six samplers had
contaminated air drawn through them at ____ mL/min for ____ min {one-quarter
of the recommended sampling time}. Sampling was discontinued and three samples set aside.
The generation system was flushed with contaminant-free air. Sampling resumed with the
other three samples having contaminant-free air {relative humidity of 80% at 22.2°C}
drawn through them at ____ mL/min for ____ h {three-quarters of the recommended
sampling time} and then all six samplers were analyzed. All of the samples in the second
set had retained more than ____% of the mean collected by the first three samples.
Low humidity
The ability of a {active sampler} to collect {analyte} from a relatively dry atmosphere was
tested by sampling an atmosphere containing ____ mg/m3 {two times the target
concentration} of {analyte} at an absolute humidity of 3.9 milligrams of water per liter of air
(about 20% relative humidity at 22.2°C). Three samplers had contaminated air drawn
through them at ____ mL/min for ____ min {the recommended sampling time}. All of the
samples were immediately analyzed. The samples had collected ____%, ____% and
____% of theoretical.
Low concentration
The ability of a {active sampler} to collect {analyte} at low concentrations was tested by
sampling an atmosphere containing ____ mg/m3 {0.1 times the target concentration} of
{analyte} at an absolute humidity of 15.7 milligrams of water per liter of air (about 80%
relative humidity at 22.2°C). Three samplers had contaminated air drawn through them
at ____ mL/min for ____ min {the recommended sampling time}. All of the samples were
immediately analyzed. The samples had collected ____%, ____% and ____% of
theoretical.
Interference
The ability of a {active sampler} to collect {analyte} was tested when other potential
interferences are present by sampling an atmosphere containing ____ mg/m3 {one times
the target concentration} of {analyte} at an absolute humidity of 15.7 milligrams of water
per liter of air (about 80% relative humidity at 22.2°C) and {interference}, whose
concentration was ____ mg/m3. Three samplers had contaminated air drawn through them
at ____ mL/min for ____ min {the recommended sampling time}. All of the samples were
immediately analyzed. The samples had collected ____%, ____% and ____% of
theoretical.
4.9.2 {Diffusive sampler}
Reverse diffusion
Table 4.9.2
Retention Efficiency (%) of {Analyte}
from {Diffusive Sampler}
|
| set |
1 | 2 |
3 | mean |
|
first
second
second/first |
49.8
50.2
100.8% |
49.1
50.1
102.0% |
50.0
50.2
100.2% |
49.6
50.2
101.2% |
|
The ability of a {diffusive sampler} to retain {analyte} after it has been collected
was tested by sampling an atmosphere containing ____ mg/m3 {two
times the target concentration} of {analyte} at an absolute humidity of 15.7 milligrams
of water per liter of air (about 80% relative humidity at 22.2°C). Six samplers were
exposed to contaminated air for ____ min {one-quarter of the recommended sampling time}.
Sampling was discontinued and three samples set aside. The generation system was flushed
with contaminant-free air. Sampling resumed with the other three samples
being exposed to humid contaminant-free air for ____ h {three-quarters of
the recommended sampling time} and then all six samplers were analyzed.
Low humidity
The ability of a {diffusive sampler} to collect {analyte} from a relatively dry atmosphere was
tested by sampling an atmosphere containing ____ mg/m3 {two times the target
concentration} of {analyte} at an absolute humidity of 3.9 milligrams of water per liter of air
(about 20% relative humidity at 22.2°C). Three samplers are exposed to contaminated
air for ____ min {the recommended sampling time}. All of the samples were immediately
analyzed. The samples had collected ____%, ____% and ____% of theoretical.
Low concentration
The ability of a {diffusive sampler} to collect {analyte} at low concentration was tested by
sampling an atmosphere containing ____ mg/m3 {0.1 times the target concentration} of
{analyte} at an absolute humidity of 15.7 milligrams of water per liter of air (about 80%
relative humidity at 22.2°C). Three samplers are exposed to contaminated air for ____
min {the recommended sampling time}. All of the samples were immediately analyzed.
The samples had collected ____%, ____% and ____% of theoretical.
Interference
The ability of a {diffusive sampler} to collect {analyte} when other potential interferences
are present was tested by sampling an atmosphere containing ____ mg/m3 {one times the
target concentration} of {analyte} at an absolute humidity of 15.7 milligrams of water per
liter of air (about 80% relative humidity at 22.2°C) and {interference}, whose concentration
was ____ mg/m3. Three samplers are exposed to contaminated air for ____ min {the
recommended sampling time}. All of the samples were immediately analyzed. The
samples had collected ____%, ____% and ____% of theoretical.
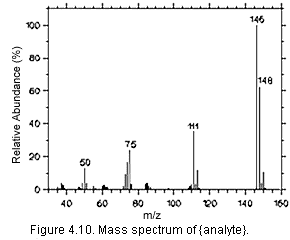
- 4.10 Qualitative analysis
{Present alternate chromatographic and GC/MS conditions that will aid in confirming
the identity of the analyte (or derivative) peak. GC/MS or LC/MS may provide the most
conclusive identification and should be addressed in all cases, even if this amounts to
an explanation why it is not possible or not available. Peak ratios and analysis with
alternate detectors may be useful. The format for mass spectrograms is shown in
Figure 4.10.}
- Partially Evaluated Methods - Data must be included on the following items:
- Background information - Include the purpose of the work, physical properties and other easily
acquired information that would normally be reported in the Background Section of a thoroughly
evaluated procedure.
- Detection limit of the overall procedure (DLOP) - Determine this parameter in the same manner as
in a thorough evaluation.
- Reliable quantitation limit - Determine this parameter in the same manner as in a thorough
evaluation.
- Extraction efficiency - Determine these parameters over the working rage of 0.5 to 2 times the target
concentration, in the same manner as in a thorough evaluation.
- Recommended sampling time and sampling rate - The recommended sampling information will at
least be based, in part, on retention efficiencies. Retention efficiencies must be performed with
loadings equivalent to twice the target concentration and with humid air (80% relative humidity at
22.2°C ).
- Storage test - In order to determine sample stability, a storage test will be performed with spiked
samples at loadings equivalent to the target concentration. This test should be performed for an
amount of storage time considered necessary. The typical age of submitted samples could be the
basis for the length of a storage test.
- Recommendation for further study - Recommendations must be made that should be considered
before a thorough evaluation is performed.
Report Partially Evaluated Methods according to the following outline. This outline is similar to that used
for an Evaluated Method except the evaluation data is included in the various appropriate method sections
instead of in a separate Backup Data section. The outline for Evaluated Methods can be a reference for
more specific format details. All Partially Evaluated Methods will have the following statement of status
on the cover page:
"Partially Evaluated Method". This method has been subjected to established evaluation procedures of
the Methods Development Team and is presented for information and trial use.
Follow the formatting information of an Evaluated Method [given on Page 17].
{ANALYTE}
{as listed in CFR or ACGIH}
| Method number: |
PV2xxx |
| |
Target concentration:
OSHA PEL:
ACGIH TLV: |
____ ppm (____ mg/m3)
____ ppm (____ mg/m3) {None if no PEL}
____ ppm (____ mg/m3) {None if no TLV} |
| |
| Procedure: |
Samples are collected by drawing workplace air through ____ with
personal sampling pumps. Samples are extracted with ____ and analyzed by ____ using a ____
detector. |
| |
| Recommended sampling time and sampling rate: |
____ min at ____ L/min (____ L) |
| |
| Reliable quantitation limit: |
____ ppm (____ mg/m3) |
| |
| Special requirements: |
{If none, delete this item} |
| |
| Status of method: |
Partially evaluated method. This method has been subjected to the established
evaluation procedures of the Methods Development Team and is presented for information and trial use. |
| |
| ____ {month year} |
{Chemist} ____ |
Chromatography Team
Industrial Hygiene Chemistry Division
OSHA Salt Lake Technical Center
Salt Lake City UT 84115-1802
-
1. General Discussion
-
- 1.1 Background
-
1.1.1 History
{Explain why past methodology is inadequate, and how the new procedure is superior.
Also, obvious questions that may be raised by knowledgeable readers should be
addressed. Keep length to 1.5 pages or less.}
1.1.2 Toxic effects (This section is for information only and should not be taken
as the basis of OSHA policy.)
{Cite sources for presented information. If both animal data and human data are
presented, present the animal data first. If the entire section is taken from one reference,
the reference notation can be placed behind the qualifying statement in the heading.}
1.1.3 Workplace exposure
{Report major sources of exposure in the workplace and, if available, the size of the work
population that is exposed. If the entire section is taken from one reference, the reference
notation can be placed behind the heading.}
1.1.4 Physical properties and other descriptive information15
| CAS number: |
____ |
vapor pressure:{kPa (mmHg)} |
____ |
| IMIS number: | ____ | l max: | ____ |
| molecular weight: | ____ | flash point: | ____ |
| boiling point: | ____ | odor: | ____ |
| melting point: | ____ | lower explosive limit: | ____ |
| appearance: | ____ | synonyms: | ____ |
| specific gravity: | ____ | structural formula: | ____ |
| molecular formula: | ____ | solubility: | ____ |
This method was evaluated according to the OSHA SLTC "EVALUATION GUIDELINES FOR AIR SAMPLING
METHODS UTILIZING CHROMATOGRAPHIC ANALYSIS".16 The Guidelines define analytical parameters,
specify required laboratory tests, statistical calculations and acceptance criteria. The analyte air
concentrations throughout this method are based on the recommended sampling and analytical parameters.
Air concentrations listed in ppm are referenced to 25°C and 101.3 kPa (760 mmHg).
-
-
1.2 Detection limit of the overall procedure (DLOP) and reliable quantitation limit (RQL)
- Example:
The DLOP is measured as mass per sample and expressed as equivalent air concentrations, based
on the recommended sampling parameters. Ten samplers were spiked with equal descending
increments of analyte, such that the highest sampler loading was ____ µg/sample. This is the
amount spiked on a sampler that would produce a peak approximately 10 times the response for
a sample blank. These spiked samplers and the sample blank were analyzed with the
recommended analytical parameters, and the data obtained used to calculate the required
parameters (standard error of estimate and slope) for the calculation of the DLOP. Values of ____
and ____ were obtained for the slope and standard error of estimate respectively. DLOP was
calculated to be ____ µg/sample (____ ppm, ____ mg/m3).
Table 1.2
Detection Limit of the Overall Procedure
|
mass per sample
(µg) |
area counts
(µV-s) |
|
0
0.421
0.841
1.262
1.682
2.103
2.524
2.944
3.365
3.785
4.206 |
0
0
178
177
375
536
696
703
810
948
1151 |
|
|
|

Figure 1.2. Plot of data to determine the DLOP/RQL. (Y = 277X - 75.8)
|
The RQL is considered the lower limit for precise quantitative measurements. It is
determined from the regression line parameters obtained for the calculation of the DLOP,
providing 75% to 125% of the analyte is recovered. The RQL is ____ µg per sample
(____ ppm, ____ µg/m3). Recovery at this concentration
is ____%.
-
2. Sampling Procedure {Refer to cited sections for format in Evaluated Methods for detail. Use paragraphs
instead of using tertiary subsections}
All safety practices that apply to the work area being sampled should be followed. The sampling
equipment should be attached to the worker in such a manner that it will not interfere with the work
performance or safety.
2.1 Apparatus {Section 2.1, [page 21]}
2.2 Reagents {If no reagents are required, state "None required". Otherwise use the format described
in Section 3.2, [page 27.]}
2.3 Technique {Section 2.3, [page 22.]}
2.4 Extraction efficiency
Example:
The extraction efficiencies of {analyte} were determined by liquid-spiking
{sampler} with the analyte at 0.5 to 2 times the target concentration. These samples were
stored overnight at ambient temperature and then extracted and analyzed. The mean
extraction efficiency over the studied range was 99.7% for {analyte}.
Table 2.4
Extraction Efficiency of {Analyte}
|
level
|
sample number
|
|
× target
concn |
µg per
sample |
1 |
2 |
3 |
4 |
mean |
|
0.25
0.5
1.0
1.5
2.0
1.0 (wet) |
105
210
421
632
841
421 |
103.5
101.6
101.9
105.8
95.8
103.5 |
99.5
102.4
101.8
105.0
92.8
99.5 |
99.6
100.5
95.8
100.4
97.7
96.6 |
100.0
95.8
100.2
94.2
97.7
100.0 |
100.6
100.1
99.9
101.4
96.0
100.7 |
|
2.5 Retention efficiency
Example:
Six {samplers} were spiked with ____ mg (____ mg/m3) {analyte}, allowed to equilibrate for 6 h, and
then had ____ L humid air (absolute humidity of 15.9 mg/L of water, about 80% relative humidity
at 22.2°C) pulled through them. The samples were extracted and analyzed. The mean retention
efficiency is ____%. There was ____% of {analyte} found on the backup portion of the {sampler}.
Table 2.5
Retention Efficiency of {Analyte}
|
|
sample number
|
|
| section | 1 | 2 | 3 | 4 | 5 | 6 | mean |
|
front
rear
total |
99.1
0
99.1 |
95.2
1.2
96.4 |
97.3
1.1
98.4 |
99.5
0
99.5 |
99.6
0
99.6 |
100.0
0
100.0 |
98.4
0.4
98.8 |
|
2.6 Sample storage
Example:
Table 2.6
Storage Test for {Analyte}
|
|
sample no.
|
| time (days) | 1 | 2 | 3 |
|
0
7
14 |
100.2
99.8
97.6 |
101.5
100.8
101.4 |
98.4
100.5
99.1 |
|
Nine {samplers} were each spiked with ____ µg (____ ppm) of {analyte}. They had
____ L of air with an absolute humidity of 15.7 milligrams of water per liter of air
(about 80% relative humidity at 22.2°C) drawn through them. They were sealed and
stored at room temperature. Three samples were analyzed immediately. Three more were
analyzed after 7 days of storage and the remaining three after 14 days of storage. The
amounts recovered, which are not corrected for extraction efficiency, indicate good storage
stability for the time period studied.
2.7 Recommended air volume and sampling rate.
Example:
Based on the data collected in this evaluation, 10-L air samples should be collected at a sampling
rate of 50 mL/min.
-
3. Analytical Procedure {Refer to cited sections of format for Evaluated Methods for
detail. Use paragraphs instead of using tertiary subsections}
Adhere to the rules set down in your Chemical Hygiene Plan17.
Avoid skin contact and inhalation of all chemicals.
- 3.1 Apparatus {Section 3.1, [page 27]}
3.2 Reagents {Section 3.2, [page 27]}
3.3 Standard preparation {Section 3.3, [page 27]}
3.4 Sample preparation {Section 3.4, [page 27]}
3.5 Analysis {Section 3.5, [page 28]}
3.6 Interferences (analytical) {Section 3.6, [page 29]}
3.7 Calculations {Section 3.7, [page 29]}
4. Recommendations for Further Study
- Studies - Report studies using the following format:
- Introduction (include purpose)
- Experimental
- Results and Discussion
- References
1 Burkhart, A.J. Appl. Ind. Hyg. 1986, 1, 153-155.
Back to text
2 Arkin, H.; Colton, R.C. Statistical Methods, 5th ed.; Barnes & Noble: New York, 1970; pp 84-88.
Back to text
3 Shulsky, M. "Review of Calculations with Solid Sorbent Passive Monitors to Determine Air Contaminent Concentrations", OSHA Salt Lake Technical Center, Salt Lake City, UT. Unpublished work, 1983.
Back to text
4 Snedcor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Iowa State University: Ames, Iowa, 1967; p 467.
Back to text
5 Arkin, H.; Colton, R.R. Statistical Methods, 5th ed.; Barnes and Noble: New York, 1970, p 85.
Back to text
6 Cassinielli, M.E.; Hull, R.D.; Crable, J.V.; and Teass, A.W., "Protocol for the Evaluation of Passive Monitors", Diffusive Sampling: An alternative Approach to Workplace Air Monitoring, Berlin, A.; Brown, R.H.; and Saunders, K.J., Eds., Royal Society of Chemistry, Burlington House, London, pp 190-202, 1987.
Back to text
7 Hendricks, W. Development of a Protocol for Laboratory Testing of Diffusive Samplers, OSHA Salt Lake Technical Center, Salt Lake City, UT. Unpublished work, 1996.
Back to text
8 Hendricks, W. Determination of the Sampling Rate Variation for SKC 575 Series Passive Samplers, OSHA Salt Lake Technical Center, Salt Lake City, UT. Unpublished work, 1998.
Back to text
9 Hendricks, W. Determination of the Sampling Rate Variation for SKC 575 Series Passive Samplers.
Back to text
10 Hendricks, W. Development of a Protocol for Laboratory Testing of Diffusive Samplers.
Back to text
11 Nelson, G.O. Gas Mixtures: Preparation and Control; Lewis: Boca Raton, 1992; Appendix M.
Back to text
12 Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.; Hendricks, W.; Rose, M.C. EVALUATION GUIDELINES FOR AIR SAMPLING METHODS UTILIZING CHROMATOGRAPHIC ANALYSIS; OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake City, UT, 1999.
Back to text
13 Occupational Exposure to Hazardous Chemicals in Laboratories. Code of Federal Regulations, Part 1910.1450, Title 29, 1998.
Back to text
14 Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.; Hendricks, W.; Rose, M.C. EVALUATION GUIDELINES FOR AIR SAMPLING METHODS UTILIZING CHROMATOGRAPHIC ANALYSIS; OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake City, UT, 1999.
Back to text
15 This reference was used for most of the physical properties.
Back to text
16 Burright, D.; Chan, Y.; Eide, M.; Elskamp, C.; Hendricks, W.; Rose, M.C. EVALUATION GUIDELINES FOR AIR SAMPLING METHODS UTILIZING CHROMATOGRAPHIC ANALYSIS; OSHA Salt Lake Technical Center, U.S. Department of Labor: Salt Lake City, UT, 1999.
Back to text
17 Occupational Exposure to Hazardous Chemicals in Laboratories. Code of Federal Regulations, Part 1910.1450, Title 29, 1998.
Back to text
|

