|
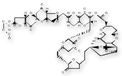

Halichondrin B and its analog, E7389.
|
Halichondrin B (NSC 609395)
E7389 (NSC 707389)
1986 Halichondrin B
(NSC 609395) is a macrocyclic polyether initially isolated from the sponge
Halichondria okadai received in 1986.
1992 It was accepted
for preclinical development by NCI in 1992 after it was found to be
highly cytotoxic in murine leukemia cells. Difficulty in collecting sufficient
material for developmental studies slowed the progress of this interesting
material.
1998 The drug received
a new lease on life with the development of a complete synthetic method
in 1998 by Dr. Yoshito Kishi of Harvard University and the discovery
that its activity resides in the macrocyclic lactone C1-C38 moiety.1
The way was now open for development of a simplified synthetic analog.
Researchers at Eisai Research Institute, who licensed the synthetic technology
from Harvard, accomplished the synthesis of the resulting drug, E7389 (NSC
707389). E7389 was presented to the DDG for preclinical development in
1998.
E7389, like its parent natural compound, is classified
as a tubulin depolymerizer, and it shows activity at least equal to the
naturally occurring chemical. It acts to disrupt the polymerization of
the microtubules necessary in mitosis.2
This general characteristic places E7389 in the group of drugs that includes
Vinca alkaloids, dolastatins, cryptophycin, and so forth, but its tubulin
interactions appear to be unique, and it was found to have greater activity
against lung and breast tumors in animal studies than either the parent
halichondrin B or paclitaxel.3
2002 E7389 entered
phase I clinical trials in 2002 and has recently progressed to phase II
clinical trials for the treatment of advanced and metastatic breast cancer.
The trials are taking place across the United States under the sponsorship
of Eisai.4
Link:
Halichondrin B
poster (pdf)
|
More than 1,100 vials of Halichondrin B were distributed
during 2004 for phase I and II clinical trials.
“DTP’s repository houses some 170,000 extracts
from samples of more than 70,000 plant and 10,000 marine organisms collected
from more than 25 countries, as well as more than 30,000 extracts of diverse
bacteria and fungi. So far, some 4,000 natural-source extracts have shown
in vitro activity against human cancer cells, making them worthy
of further study by DTP researchers looking to find nature’s next
blockbuster drug.”5
—David Newman, Ph.D., Natural Products Branch,
NCI
|



