|
<<< Back to Sampling and Analytical Methods |
 Printing Instructions
Printing Instructions |
For problems with accessibility in using figures, illustrations and PDFs in this method, please contact
the SLTC at (801) 233-4900. These procedures were designed and tested for internal use by OSHA personnel.
Mention of any company name or commercial product does not constitute endorsement by OSHA. |
Methotrexate
[152 KB PDF,
7 pages]
Related Information: Chemical Sampling -
Methotrexate
|
| Matrix: |
Air |
|
|
| Control no.: |
T-PV2146-01-8804-Ch |
|
|
| Target Concentration: |
0.04 mg/m3 (arbitrary). There
is no OSHA PEL or AGGIH TLV for methotrexate. |
|
|
| Procedure: |
Samples are collected by
drawing known volumes of air through OSHA versatile sampling tubes (OVS-2)
containing a glass fiber filter and two sections of XAD-2 adsorbent. Samples
are desorbed with a solution of 0.4 M sodium carbonate in 25% methanol/75%
water (v/v) and analyzed by high performance-liquid chromatography (HPLC)
using an ultraviolet (UV) detector. |
|
|
Recommended air volume
and sampling rate: |
120 L at 1 L/min
|
|
|
| Detection limit
of-the overall procedure (based on the recommended air volume): |
0.00015 mg/m3 |
|
|
| Status of method: |
Partially evaluated method.
This method has been partially evaluated and is presented for information
and trial use. |
|
|
| Date: April 1988 (final) |
Mary E. Eide |
|
|
Carcinogen and Pesticide Branch
OSHA Analytical Laboratory
Sandy UT 84070-6406 |
1. General discussion
1.1. Background
The OSHA Analytical Laboratory received a set of samples with a request for the analysis of methotrexate. The samples had been collected on OVS-2 sampling tubes at a flow rate of 1 liter per minute.
This report describes the preliminary validation of the sampling method and the development of an analytical method for methotrexate.
An OSHA in-house Stopgap Method was previously developed for methotrexate (Reference 5.1) that specifies glass fiber filters as the collection medium. This work was performed to gather additional
evaluation data.
1.2. Toxic effects (This section is for information only and should not be taken as the basis of OSHA policy.)
Data on mutation, reproductive effects, tumorigenic, and toxicity are summarized in
Reference 5.4.
1.3. Potential workplace exposure
Methotrexate is an antineoplastic agent used in the treatment of acute lymphoblastic leukemia, non-Hodgkin's lymphoma, osteogenic sarcoma-, synovial sarcoma, breast cancer, embryonal
rhabdomyo¬sarcoma, testicular tumors, carcinomas of the lung and uterine cervix, squamous-cell carcinoma of the head and neck, various soft-tissue sarcomas, mycosis fungoides, histiocytosis and
solitary plasmacytoma (Reference 5.3).
The workers liable for workplace exposure are those handling the antineoplastie agents at the hospitals.
1.4.Physical properties and other descriptive information
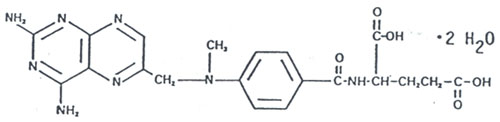
| Chemical name: |
1-Glutamic acid,
N-(4-(((2,4-diamino-6-pteridinyl)methyl)methylamino))benzoyl |
| IMIS no.: |
M106 |
| IUPAC name: |
1-(+)-N-(p-(((2,4-Diamino-6-pteridinyl)¬methyl)methylamino)benzoyl)glutamic
acid |
| Synonyms: |
Amethopterin
4-Amino-l0-methylfolic acid
Methotrexatum
Alpha-methopterin
Hethylaminopterin |
| Trade names: |
A-Methopterin
Antifolan
CL-14377
Ledertrexate
Mthotrexate specia
MEXATE
MYX
NSC-740
R 9985 |
| CAS no.: |
59-05-2 |
| Molecular formula: |
C20H22N805 |
| Molecular weight: |
454.4 |
| Structure: |
 |
| Appearance: |
Bright yellow-orange, odorless, crystalline
powder |
| Melting point: |
185-204 °C (monohydrate) |
| UV max: |
307, 243 nm (in 0.1 N HCl)
303, 258, 372 nm (in 0.1 N NaOH) |
| Solubility: |
Practically insoluble in water, ethanol,
chloroform and diethyl ether; freely soluble in dilute solutions of alkaline
hydroxides and carbonates; soluble in dilute hydrochloric acid. |
| Stability: |
Sensitive to hydrolysis, oxidation and
light. |
1.5 Detection limit of the analytical procedure
The detection limit of the analytical procedure is 0.44 ng per injection. This is the amount of analyte which will give a peak whose height is approximately 5 times the baseline noise.
2. Sampling procedure
2.1. Apparatus and reagents
2.1.1. A personal sampling pump that can be calibrated to within ±5% of the recommended flow rate.
2.1.2. OVS-2 sampling tube, containing glass fiber filter and two sections of XAD-2 adsorbent.
2.2. Technique (Reference 5.2)
2.2.1. Calibrate pump at 1 L/min.
2.2.2. Attach the collection device to the shirt collar or within the breathing zone. Position the excess tubing so as not to interfere with the work of the employee.
2.2.3. Turn on pump and record the starting time.
2.2.4. Check the pump flow periodically.
2.2.5. Prepare a blank. Handle the blank the same as the other samples but do not draw air through it.
2.2.6. At the end of the sampling period, turn off the pump and record the ending time.
2.2.7. Cap and seal the sampling tube with a Form OSHA-21.
2.3. Recommended air volume and sampling rate
2.3.1. The recommended air volume is 120 L.
2.3.2. The recommended sampling rate is 1 L/min.
2.4. Extraction efficiency
Three OVS-2 sampling tubes were each liquid spiked with 4.4 μg of methotrexate. The samples were treated as in Section 3.4. and analyzed. The average recovery was 87.2%.
| Extraction
Efficiency |
| sample |
recovered (μg) |
recovery (%) |
| YC 4 |
3.84 |
87.3 |
| YC 5 |
3.85 |
87.5 |
| YC 6 |
3.82 |
86.8 |
| |
average |
87.2 |
2.5. Retention efficiency
Three OVS-2 sampling tubes were each liquid spiked with 4.4 μg of methotrexate. Humid air (70% RH, 120 L at 1 L/min) was drawn through the sampling tubes. The samples were treated as in section
3.4.
and analyzed. The average recovery was 83.3%.
| Retention
Efficiency |
| sample |
recovered (μg) |
recovery (%) |
| YC 7 |
3.52 |
80.0 |
| YC 8 |
3.76 |
85.5 |
| YC 9 |
3.72 |
84 |
| |
average |
83.3 |
2.6. Sample storage
Three OVS-2 sampling tubes were each liquid spiked with 4.4 μg of methotrexate. Humid air (70% RH, 120 L at 1 L/min) was drawn through the sampling tubes. They were stored at room temperature
in the
dark for 6 days, treated as in section 3.4. and analyzed. The average recovery was 82.4%.
| Sample Storage |
| sample |
recovered (μg) |
recovery (%) |
| YC 10 |
3.63 |
82.5 |
| YC 11 |
3.65 |
83.0 |
| YC 12 |
3.60 |
81.8 |
| |
average |
82.4 |
2.7. Interferences
There are no known interferences to the sampling procedure.
3. Analytical method
3.1. Apparatus
3.1.1. High performance liquid chromatograph
3.1.2. Chromasil 5 μm C18 column or equivalent
3.1.3. UV detector
3.1.4. Stripchart recorder
3.2. Reagents
3.2.1. Water, HPLC grade
3.2.2. Methanol, HPLC grade
3.2.3. 1-(+)-Amethopterin dihydrate (Methotrexate), 98% (Aldrich)
3.2.4. Sodium phosphate, dibasic, reagent grade
3.2.5. Phosphoric acid, reagent grade
3.2.6. 0.05 M Sodium carbonate aqueous solution
3.3. Standard preparation
Weigh 4 to 8 mg of methotrexate in a 10-mL volumetric flask. Add 0.05 M sodium carbonate aqueous solution to the mark. Dilute with 0.05 M sodium carbonate solution to a suitable working range.
3.4. Sample preparation
3.4.1. Transfer the glass fiber filter and the front section of the XAD-2 to a 4-mL vial. Place the separating foam plug and the back section of the XAD-2 in a separate vial.
3.4.2. Add 4.0 mL of a solution of 0.04 M sodium carbonate in 25% methanol/75% water (v/v) to each vial.
3.4.3. Seal the vials with PTFE-lined caps and shake for 30 minutes on a mechanical shaker.
3.5. Analysis
3.5.1. Instrument conditions
| Column: |
Chromasil 5 μm C18 |
| Eluent: |
20% Acetonitrile, 80% water, 0.04 M
phosphate buffer, pH 6.7 |
| Flow rate: |
1.0 mL/min |
| Detector: |
303 nm (primary), 258 nm |
| Injection size: |
100 μL |
| Retention time: |
3.8 min |
3.5.2. Chromatogram (see Figure 1)
3.6. Interferences (Analytical)
3.6.1. Any collected compound that has the same retention time as methotrexate and absorbs at 303 and 258 nm is a potential interference. Generally, chromatographic conditions can be altered to
separate an interference from the analyte.
3.6.2. Retention time alone is not proof of chemical identity. Confirmation by other means should be sought whenever possible.
3.7. Calculations
3.7.1. A calibration curve is constructed by plotting standard concentrations versus detector response (see Figure 2).
3.7.2. The concentration of a sample is determined from the calibration curve.
3.7.3. The air concentration is determined by the following formula:
| mg/m3 = |
μg/mL x 4 mL |
|
| air volume (L) x (decimal
equivalent of extraction efficiency) |
3.8. Safety precautions (Analytical)
3.8.1. Avoid exposure to standards and solvents.
3.8.2. Wear safety glasses.
4. Recommendations for further study
4.1. The method should be fully validated.
 |
Figure 1. Chromatogram of Methotrexate at 303 nm.
|
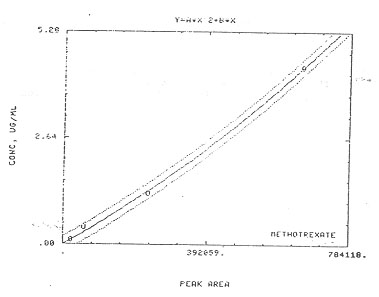
Figure 2. Calibration Curve of Methotrexate |
5. References
5.1. Armitage, D.B., "Methotrexate", Stopgap Method, U.S. Department of Labor, OSHA Analytical Laboratory, 1983.
5.2. "Industrial Hygiene Technical Manual", OSHA Instruction CPL; 2-2.20a, March 30, 1984, U.S. Department of Labor. Chapter II: "Standard Methods for Sampling Air Contaminants."
5.3. WHO, "IARC Monograph on the Evaluation of the Carcinogenic Risk of Chemicals to Man", Volume 26; International Agency for Research on Cancer: Lyon, 1981, pp. 267-293.
5.4. "Registry of Toxic Effects of Chemical Substances, 1983-84 Supplement"; U.S. Department of Health and Human Services: Cincinnati, OH, 1986; DHHS (NIOSH) Publication no. 86-103.
|
| |
|

