NINDS 2009 Congressional Justification
Skip secondary menuDEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute of Neurological Disorders and Stroke
2009 Fiscal Year Budget
Congressional Justification
- Organization Chart
- Appropriation Language
- Amounts Available for Obligation
- Budget Mechanism Table
- Budget Authority by Program
- Major Changes in Budget Request
- Summary of Changes
- Budget Graphs
- Justification Narrative
- Budget Authority by Object
- Salaries and Expenses
- Authorizing Legislation
- Appropriations History
- Detail of Full-Time Equivalent Employment (FTE)
- Detail of Positions
- New Positions Requested
Organization Chart
Appropriation Language
For carrying out section 301 and title IV of the Public Health Services Act with respect to neurological disorders and stroke, $1,571,353,000 $1,545,397,000 (Department of Health and Human Services Appropriation Act, 2008)
Amounts Available for Obligation 1/
| Source of Funding | F Y 2007 Actual | F Y 2008 Enacted | F Y 2009 Estimate |
|---|---|---|---|
| Appropriation | $1,534,757,000 | $1,571,353,000 | $1,545,397,000 |
| Pay cost add-on | 78,000 | 0 | 0 |
| Rescission | 0 | -27,452,000 | 0 |
| Subtotal, adjusted appropriation | 1,535,545,000 | 1,543,901,000 | 1,545,397,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | -2,557,000 | 0 | 0 |
| Real transfer to the Global Fund to fight HIV/AIDS, Malaria and Tuberculosis | 0 | 0 | 0 |
| Real transfer to the Office of Public Health Emergency Preparedness | 0 | 0 | 0 |
| Comparative transfer to NIBIB | -71,000 | 0 | 0 |
| Comparative transfer to OD | -32,000 | 0 | 0 |
| Comparative transfer to NCRR | -535,000 | 0 | 0 |
| Comparative transfers to the Office of the Assistant Secretary for Admin. and Mgmt. and to the Office of the Assistant Secretary for Public Affairs | -3,000 | 0 | 0 |
| Comparative transfer to (specify) | 0 | 0 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | 2,557,000 | 0 | 0 |
| Comparative transfer to the Global Fund to fight HIV/AIDS, Malaria and Tuberculosis | 0 | 0 | 0 |
| Comparative transfer from DHHS | 0 | 0 | |
| Comparative transfer to DHHS for PHS historian | 0 | 0 | |
| Subtotal, adjusted budget authority | 1,534,904,000 | 1,543,901,000 | 1,545,397,000 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
| Subtotal, adjusted budget authority | 1,534,904,000 | 1,543,901,000 | 1,545,397,000 |
| Unobligated balance lapsing | -11,000 | 0 | 0 |
| Total obligations | 1,534,893,000 | 1,543,901,000 | 1,545,397,000 |
1/ Excludes the following amounts for reimbursable activities carried out by this account: FY 2007 - $8,352,799 FY 2008 - $12,125,000 FY 2009 - $12,125,000 Excludes $411,103 in FY 2008 and $417,972 in FY 2009 for royalties.
Budget mechanism table
| MECHANISM | FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimate |
Change | ||||
|---|---|---|---|---|---|---|---|---|
| Research Grants: | Number | Amount | Number | Amount | Number | Amount | Number | Amount |
| Research Projects: | ||||||||
| Noncompeting | 2,050 | $796,537 | 1,894 | $757,760 | 1,949 | $765,307 | 55 | $7,547 |
| Administrative supplements | (104) | 6,485 | (104) | 6,485 | (104) | 6,485 | (0) | 0 |
| Competing: | ||||||||
| Renewal | 174 | 85,439 | 200 | 99,332 | 193 | 95,891 | (7) | -3,441 |
| New | 473 | 143,420 | 545 | 166,740 | 526 | 160,965 | (19) | -5,775 |
| Supplements | 1 | 75 | 1 | 87 | 1 | 84 | 0 | -3 |
| Subtotal, competing | 648 | 228,934 | 746 | 266,159 | 720 | 256,940 | (26) | -9,219 |
| Subtotal, RPGs | 2,698 | 1,031,956 | 2,640 | 1,030,404 | 2,669 | 1,028,732 | 29 | -1,672 |
SBIR/STTR |
99 | 37,675 | 97 | 36,701 | 97 | 36,653 | 0 | -48 |
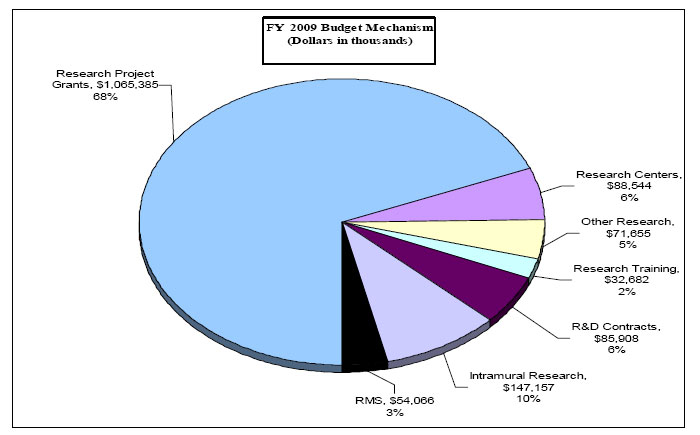
| Subtotal, RPGs | 2,797 | 1,069,631 | 2,737 | 1,067,105 | 2,766 | 1,065,385 | 29 | -1,720 |
Research Centers: |
||||||||
| Specialized/comprehensive | 69 | 87,552 | 70 | 88,544 | 70 | 88,544 | 0 | 0 |
| Clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Centers | 69 | 87,552 | 70 | 88,544 | 70 | 88,544 | 0 | 0 |
Other Research: |
||||||||
| Research careers | 296 | 47,354 | 320 | 49,061 | 320 | 49,061 | 0 | 0 |
| Cancer education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative clinical research | 63 | 5951 | 64 | 6,018 | 64 | 6,018 | 0 | 0 |
| Biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority biomedical research support | 4 | 1,202 | 4 | 1,202 | 4 | 1.202 | 0 | 0 |
| Other | 66 | 15,202 | 67 | 15,374 | 67 | 15,374 | 0 | 0 |
| Subtotal, Other Research | 429 | 69,696 | 455 | 71,655 | 455 | 71,655 | 0 | 0 |
| Total Research Grants | 3,295 | 1,226,879 | 3,262 | 1,227,304 | 3,291 | 1,225,584 | 29 | -1,720 |
Research Training: |
F T T P's |
F T T P's |
F T T P's |
|||||
| Individual awards | 411 | 15,390 | 411 | 15,390 | 411 | 15,513 | 0 | 123 |
| Institutional awards | 346 | 17,050 | 346 | 17,050 | 346 | 17,169 | 0 | 119 |
| Total, Training | 757 | 32,440 | 757 | 32,440 | 757 | 32,682 | 0 | |
Research & development contracts |
85 | 81,223 | 103 | 85,908 | 103 | 85,908 | 0 | 0 |
| (SBIR/STTR) | (0) | (69) | (0) | (69) | (0) | (69) | (0) | (0) |
| F T E's | F T E's | F T E's | F T E's | |||||
| Intramural research | 364 | 142,139 | 364 | 144,982 | 370 | 147,157 | 6 | 2,175 |
| Research management and support | 153 | 52,223 | 153 | 53,267 | 155 | 54,066 | 2 | 799 |
| Construction | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Buildings and Facilities | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total, NINDS | 517 | 1,534,904 | 517 | 1,543,901 | 525 | 1,545,397 | 8 | 1,496 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Budget Authority by Program
| FY 2005 Actual |
FY 2006 Actual |
FY 2007 Actual |
FY 2007 Comparable |
FY 2008 Enacted |
FY 2009 Estimated |
Change |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: |
FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Channels, Synapses & Circuits | $230,197 | $223,197 | $193,083 | $193,375 | $194,112 | $193,899 | -$213 | |||||||
| Neural Environment | 338,930 | 333,020 | 330,855 | 331,354 | 332,617 | 332,252 | -365 | |||||||
| Neurodegeneration | 197,778 | 186,827 | 192,755 | 193,046 | 193,782 | 193,569 | -213 | |||||||
| Neurogenetics | 218,562 | 205,461 | 211,039 | 211,358 | 212,164 | 211,931 | -233 | |||||||
| Repair & Plasticity | 136,052 | 141,135 | 154,237 | 154,470 | 155,059 | 154,889 | -170 | |||||||
| Neuroscience | 195,378 | 189,691 | 191,996 | 192,286 | 193,019 | 192,807 | -212 | |||||||
| Technology Development, Infrastructure & Resources |
41,937 | 60,709 | 64,555 | 64,653 | 64,899 | 64,827 | -72 | |||||||
Subtotal, Extramural |
1,358,834 | 1,339,970 | 1,338,520 | 1,340,542 | 1,345,652 | 1,344,174 | -1,478 | |||||||
Intramural research |
385 | 134,617 | 374 | 142,648 | 364 | 142,234 | 364 | 142,139 | 364 | 144,982 | 370 | 147,157 | 6 | 2,175 |
Res. management & support |
146 | 45,997 | 152 | 51,085 | 153 | 52,234 | 153 | 52,223 | 153 | 53,267 | 155 | 54,066 | 2 | 799 |
TOTAL |
531 | 1,539,448 | 526 | 1,533,703 | 517 | 1,532,988 | 517 | 1,534,904 | 517 | 1,543,901 | 525 | 1,545,397 | 8 | 1,496 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Major Changes in the Fiscal Year 2009 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2009 budget request for NINDS, which is $1.496 million more than the FY 2008 Enacted, for a total of $1,545.397 million.
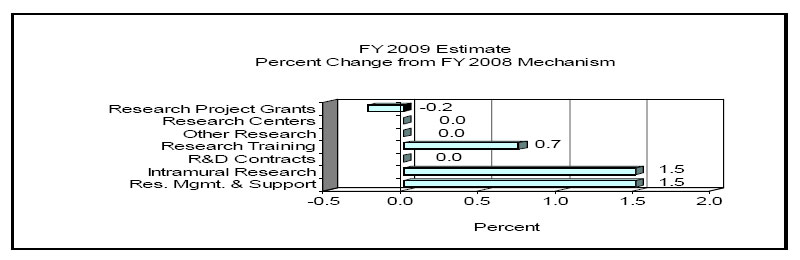
Research Project Grants (-1.672 million, total $1,028.7 million) NINDS will support a total of 2,669 Research Project Grant (RPG) awards in FY 2009. Noncompeting RPGs will increase by 55 awards and increase by $7.547 million. Competing RPGs will decrease by 26 awards and decrease by $9.219 million. The NIH Budget policy for RPGs in FY 2009 does not include inflationary increases in noncompeting awards or increases in average costs for competing grants.
Intramural Research - Neurogenetics Branch (+$2 million; total $8.3 million): NINDS will expand the intramural Neurogenetics Branch with the addition of two tenure track investigators to pursue exceptional opportunities that are emerging for progress toward therapies for neurogenetic disorders.
Intramural Research - Neuronal Cell Biology (+$1 million; total $1 million): NINDS will expand the intramural program in cell biology, a rapidly advancing area of research that has implications for many neurological diseases.
NINDS Clinical Research Consortium (CRC) (+$2 million; total $2 million): Based on early experience, NINDS is revamping the CRC to more effectively meet its goals of increasing patient access to and recruitment to clinical trials through involvement of community neurologists.
Summary of changes
| F Y 2008 enacted | $1,543,901,000 | |||
|---|---|---|---|---|
| F Y 2009 estimated budget authority | 1,545,397,000 | |||
| Net change | 1,496,000 | |||
| 2008 Current Enacted Base |
Change from Base | |||
| Budget | Budget | |||
| CHANGES | F T E's | Authority | F T E's | Authority |
| A. Built-in: 1. Intramural research: |
||||
| a. Annualization of January 2008 pay increase |
$51,307,000 | $576,000 | ||
| b. January F Y 2009 pay increase | 51,307,000 | 1,116,000 | ||
| c. One less day of pay | 51,307,000 | (204,000) | ||
| d. Payment for centrally furnished services | 26,129,000 | 392,000 | ||
| e. Increased cost of laboratory supplies, materials, and other expenses |
67,546,000 | 1,206,000 | ||
| Subtotal | 3,086,000 | |||
2. Research management and support: |
||||
| a. Annualization of January 2008 pay increase |
$20,517 | $230,000 | ||
| b. January F Y 2009 pay increase | 20,517 | 446,000 | ||
| c. One less day of pay | 20,517 | (82,000) | ||
| d. Payment for centrally furnished services | 11,411 | 171,000 | ||
| e. Increased cost of laboratory supplies, materials, and other expenses |
21,339 | 399,000 | ||
| Subtotal | 1,164,000 | |||
Subtotal, Built-in |
4,250,000 | |||
| 2008 Current | ||||
|---|---|---|---|---|
| Enacted Base | Change from Base | |||
| CHANGES | No. | Amount | No. | Amount |
| B. Program: | ||||
| 1. Research project grants: | ||||
| a. Noncompeting | 1,894 | $764,245,000 | 55 | $7,547,000 |
| b. Competing | 746 | 266,159,000 | (26) | (9,219,000) |
| c. SBIR/STTR | 97 | 36,701,000 | 0 | (48,000) |
| Total | 2,737 | 1,067,105,000 | 29 | (1,720,000) |
| 2. Research centers | 70 | 88,544,000 | 0 | 0 |
| 3. Other research | 455 | 71,655,000 | 0 | 0 |
| 4. Research training | 757 | 32,440,000 | 0 | 242,000 |
| 5. Research and development contracts | 103 | 85,908,000 | 0 | 0 |
| Subtotal, extramural | (1,478,000) | |||
| F T E's | F T E's | |||
| 6. Intramural research | 364 | 144,982,000 | 6 | (911,000) |
| 7. Research management and support | 153 | 53,267,000 | 2 | (365,000) |
| 8. Construction | 0 | 0 | ||
| 9. Buildings and Facilities | 0 | 0 | ||
| Subtotal, program | 1,543,901,000 | (2,754,000) | ||
| Total changes | 517 | 8 | 1,496,000 | |
Budget Graphs
History of Budget Authority and FTE's:
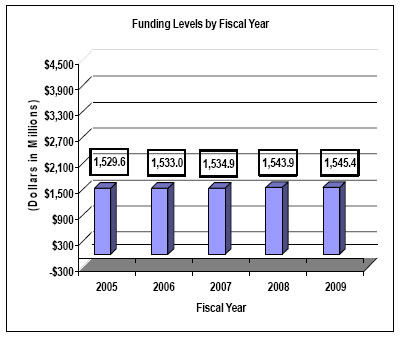
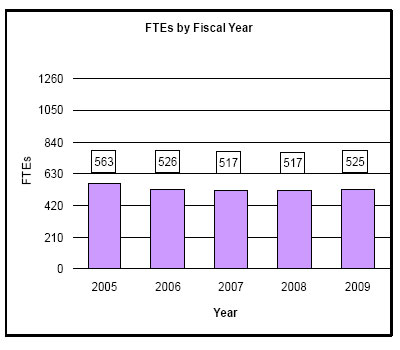
Distribution by Mechanism:
Change by Selected Mechanisms:
Justification
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.| FY 2007 Actual |
FY 2008 Enacted |
FY 2009 Estimated |
Increase or Decrease |
||||
|---|---|---|---|---|---|---|---|
| F T E's | B A | F T E's | B A | F T E's | B A | F T E's | B A |
| 517 | 1,532,988,000 | 517 | $1,532,901,000 | 525 | $1,545,397,000 | 8 | $1,496,000 |
This document provides justification for the Fiscal Year (FY) 2009 activities of the National Institute of Neurological Disorders and Stroke (NINDS), including NIH/AIDS activities. Details of the FY 2009 HIV/AIDS activities are in the "Office of AIDS Research (OAR)" Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director's Overview
NINDS has launched a strategic planning process that will examine how the institute can better carry out its mission. The process will include all stakeholders in discussions of basic, translational, and clinical research across the spectrum of neurological disorders. To set the vision that will frame these pragmatic discussions, NINDS began by asking stakeholders to suggest "blue sky" goals to which NINDS should aspire.
In considering the future, it is useful to look at the past. Because of progress over the last 15 to 20 years, hundreds of thousands of strokes are prevented each year, the first effective emergency treatment can improve the outcome for people who do have a stroke, and science-based stroke rehabilitation is emerging. According to the Centers for Disease Control and Prevention, the age adjusted stroke death rate is continuing to decline, from 65.3 per 100,000 population in 1990 to 46.6 in 2005. Progress is also apparent for other neurological disorders; better drugs and surgical treatments now help people who have chronic pain, epilepsy, migraine, multiple sclerosis, neuropathies, Parkinson's disease, and many other diseases. Advances in brain imaging and in genetic testing have transformed diagnosis and treatment of many neurological diseases. Although the challenges remain formidable, we have entered an era in which neurologists and neurosurgeons can prevent and treat, in addition to diagnosing, many disorders.
The prospects for the future are also encouraging. With the discovery of gene defects that cause hundreds of inherited disorders, scientists can now work to understand what goes wrong in the brain, generate animal models that mimic human disease, and rationally develop therapies. Researchers have also begun to identify how genes contribute to the susceptibility and progression of common diseases, including stoke, multiple sclerosis, and Parkinson's disease, and are working to understand how genes and environment together cause disease. Building on basic research advances, NIH and private sector research teams are developing drugs that precisely target critical steps in the disease process, stem cell and regenerative therapies that repair damage, devices that adjust malfunctioning brain circuits, therapies that silence or replace defective genes, and behavioral interventions that encourage the "plasticity" of the brain and spinal cord to compensate for damage. In the future, insights from neuroscience will not only treat disease after it occurs, but also help people avoid disease and maintain healthy brains throughout their lifespan.
To continue progress, NINDS Intramural investigators conduct research on the NIH campus in Bethesda, Maryland, and the extramural program supports researchers at universities, medical centers, and small businesses throughout the U.S. Although the Institute targeted initiatives to specific problems when appropriate, NINDS emphasizes investigator-initiated research, which is especially suited to the diversity of challenges that nervous system diseases present. Investigators respond to institute priorities, because it engages the research community in identifying needs and opportunities. In 2007, for example, a major conference assessed progress since the 2000 conference that launched the Epilepsy Benchmarks planning process, and the research and patient community are working with NINDS to update the benchmarks. Other recent scientific workshops focused on ataxia-telangiectasia, frontotemporal dementia, gene therapy, hydrocephalus, mucopolysaccharidoses, muscular dystrophies, neonatal seizures, pain, Parkinson's disease, peripheral neuropathies including Charcot Marie Tooth disorder, spinocerebellar ataxias, stroke, and traumatic brain injury, among other topics. Ongoing NINDS extramural programs encourage the scientific community to seek out and exploit opportunities for basic, translational, and clinical research on all neurological disorders, and provide many ways for researchers to collaborate toward common goals.
NIH training of researchers, like NIH basic research, enhances progress in both the public and private sectors. NINDS has extended the payline for new investigators and supported the NIH Pathway to Independence Awards. The Institute continues its programs to promote diversity. Innovation is also crucial. The Institute supports NIH Pioneer Awardees and in 2008 began participation in the new EUREKA (Exceptional, Unconventional Research Enabling Knowledge Acceleration) program, which complements the NIH-wide Pioneer and New Innovator Award programs. In 2009, NINDS will continue to balance investigator-initiated research with initiatives that target opportunities that emerge from the strategic and disease planning efforts. New and continuing initiatives planned for 2009 will focus on:
- Renewal of the Cooperative Program in Translational Research, which supports teams of researchers to develop therapies toward readiness for clinical testing. This program is showing encouraging progress, with projects moving towards clinical trials.
- Research on axon (nerve fiber) damage in multiple sclerosis and on how immune cells contribute, in beneficial and harmful ways, to many nervous system disorders, including multiple sclerosis.
- The NINDS Neural Prosthesis Program, which for more than three decades has pioneered the development of devices that restore or supplement nervous system functions lost by disease or damage.
- Brain mechanisms of functional recovery after stroke. Stroke rehabilitation based on understanding of brain plasticity is showing encouraging results in clinical trials.
- The CAPTR (Collaborative Activities to Promote Translational Research) administrative supplement program, which encourages investigators to develop new interdisciplinary collaborations that lead to therapy development.
- The next phase of the ImPACT (Immediate Practice Altering Clinical Trials) program. ImPACT is developing a computer model to estimate the cost-benefits of proposed clinical trials and will test the validity and usefulness of the model within the Institute's strategic planning process.
- Solicitations also continue in 2009 on ataxia telangiectasia, autism, bioengineering, deep brain stimulation, developmental pharmacology, dystonias, HIV infection in the central nervous system, migraine, neuroimaging, sarcoidosis, and women's health, among other topics. Many are cooperative efforts among NIH institutes and centers.
The NINDS participates in the support of the Translational Research Core Service Support and the Assay Technology Development initiatives funded through the NIH Common Fund.
FY2009 Justification by Activity Detail
Overall Budget Policy:
The NINDS continues to place high priority on supporting investigator-initiated research projects, new investigator research, and career development. NINDS maintains a balance between funding made available to support investigator-initiated projects and targeted solicitations issued to the extramural community in areas that need stimulation. The Institute carefully evaluates the mission relevance of requests to submit applications for all large investigator-initiated projects and of all Institute initiatives. Beginning in 2007, NINDS has set a maximum of 10 years duration for all unsolicited program project grants. Intramural Research and Research Management and Support receive modest increases to help offset the cost of pay and other increases. NINDS will continue to support new investigators and to maintain an adequate number of competing RPGs.
Program Descriptions and Accomplishments
Channels, Synapses, and Circuits
Ion channels, synapses, and neural circuits are fundamental elements of the nervous system. NINDS supports research on how these elements operate in the healthy nervous system in the adult and developing brain and on diseases in which they play a central role, including epilepsy. In March 2007, NINDS, working closely with non-governmental organizations, held a major conference "Curing Epilepsy II." The conference assessed progress against the Epilepsy Benchmarks that arose from the first "Curing Epilepsy" conference in 2000. Progress has been encouraging and the Institute is working with the epilepsy research and patient communities to update those benchmarks to reflect new goals that will guide future research.
Budget Policy: The 2009 budget estimate of $193,899,000 for Channels, Synapses, and Circuits represents a decrease of $213,000 from the 2008 enacted. In 2009, NINDS will continue to balance investigator-initiated and solicited research, including projects funded through the Institute's broad translational research and clinical trials programs. The NINDS is participating in an NIH program announcement, active through 2009, on the structural biology of membrane proteins, which include ion channels, neurotransmitter receptors, and other proteins that are critical in epilepsy and other neurological disorders.
Neural Environment
Fewer than 1 of 10 cells in the human brain are nerve cells. The non-neuronal cells maintain the local environment in the brain that surrounds nerve cells, fight infections, control which molecules in the blood circulation get into the brain, and serve the healthy and diseased brain in many other ways. These cells play a central role in several diseases that affect children and adults, including stroke, multiple sclerosis, brain tumors, neurofibromatosis, tuberous sclerosis, transmissible spongiform encephalopathies, neuroAIDs, and other infectious diseases. NINDS supports a wide spectrum of research on non-neuronal cells in the healthy and diseased brain and on translating scientific knowledge into better diagnosis, prevention, and treatment, including drug delivery to the brain. In 2007, the Stroke Progress Review Group (PRG) reported a major mid-course review of stroke research. The detailed reports will help guide the extensive NINDS stroke research programs. Program announcements continuing in 2008 focus on understanding and treating tuberous sclerosis complex and on transmissible spongiform encephalopathies, including Creutzfeldt-Jakob disease.
Budget Policy: The 2009 budget estimate of$322,252,000 for Neural Environment represents a decrease of $365,000 from the 2008 enacted. NINDS will continue to rely on a balance of solicited and investigator-initiated research, including research through the Institute's broad translational research and clinical trials programs. New NINDS solicitations in 2009 will focus on understanding and treating axon (nerve fiber) damage in multiple sclerosis, on the trafficking of immune cells into the brain and spinal cord, and on brain mechanisms that lead to functional recovery after stroke. In 2009, NINDS is also participating in a National Cancer Institute led initiative on adult brain tumor and in NIH initiatives begun in previous years that focus on HIV in the central nervous system, on sarcoidosis, and on sleep disorders.
Neurodegeneration
Alzheimer's disease, amyotrophic lateral sclerosis (ALS), frontotemporal dementias, Huntington's disease, and Parkinson's disease are among the neurodegenerative diseases that affect adults. For many neurodegenerative disorders, risk increases with age. So, these diseases present an increasing challenge to the U.S. as our population distribution ages. NINDS neurodegeneration research priorities include understanding how genes and the environment cause neurodegeneration and translating those insights into treatment and prevention. Among program activities in 2007, an independent review confirmed the value of the Morris K. Udall Parkinson's Disease Centers of Excellence and provided guidance that will be valuable for improving the continuing program; the NIH Exploratory Trials in Parkinson's Disease (NET-PD) program launched its first large multi-site clinical trial of a drug to slow the progression of Parkinson's disease; a scientific workshop examined the current status of research and opportunities for frontotemportal dementia; and a solicitation on the cognitive problems of Parkinson's disease is active through 2008.
Budget Policy: The 2009 budget estimate for Neurodegeneration activities is $322,252,000, a decrease of $213,000 from the FY 2008 enacted. NINDS neurodegeneration research will continue to balance investigator-initiated research and solicited research, including projects funded through the Institute's broad translational research and clinical trials programs. Neurodegeneration program efforts continuing in 2009 include the Morris K. Udall Parkinson's Disease Centers of Excellence, the CINAPS (Committee to Identify Neuroprotective Agents for Parkinson's disease) program, the NIH Exploratory Trials in Parkinson's Disease (NET-PD), and other clinical trials. The Institute is participating in an NIH Alzheimer's disease drug discovery initiative that is continuing in 2009.
Neurogenetics
Gene defects directly cause hundreds of diseases that affect the nervous system at all stages of development, from infancy to adulthood. Gene variations among individuals also contribute to the susceptibility and progression of most common neurological disorders. Neurogenetic disorders include the ataxias, Down syndrome, dystonia, lysosomal storage diseases (which include Batten disease and the mucopolysaccharidoses), muscular dystrophies, inherited peripheral neuropathies, Rett syndrome, and Tourette syndrome, among many others. NINDS supports research to understand genes and the nervous system, which often provide key insights into both inherited and non-inherited disorders, and to develop gene-based treatments, which similarly have enormous potential for many diseases. Recent activities include scientific workshops on mucopolysaccharidoses, ataxia-telangiectasia, peripheral neuropathies, including Charcot Marie Tooth disorder, Down syndrome, and gene therapy in the nervous system. Solicitations that are active through 2008 focus on Rett syndrome, muscular dystrophy, lysosomal storage disorders, and RNAi therapy, which has promise for silencing harmful genes in several neurological disorders.
Budget Policy: The 2009 budget estimate of $211,931,000 for Neurogenetics represents a decrease of $233,000 from the 2008 enacted. NINDS will continue investigator initiated grants and targeted activities in neurogenetics, including projects funded through the Institute's broad translational research and clinical trials programs. Program announcements that continue through 2009 solicit research on generalized and focal dystonias, on fragile X syndrome and autism, and on priorities of the ataxia telangiectasia (A-T) research plan, which the NIH developed in 2006 in cooperation with non-governmental organizations.
Repair and Plasticity
NINDS supports extensive research on spinal cord injury and traumatic brain injury, and on repairing damage to the nervous system from disease or trauma. Research on repair includes longstanding support for the study of neural stem cells, of the brain's innate capacity to adapt through "plasticity", and of neural prostheses, which are devices that restore function. In recent years, stimulated by the high rate of traumatic brain injury among U.S. military personnel, NINDS has enhanced coordination of traumatic brain injury research with the several Federal Agencies that support such research, including the Departments of Defense and Veterans Affairs. In 2008, NINDS is renewing funding for the Facilities of Research Excellence in Spinal Cord Injury (FORE SCI) program, which address critical needs for spinal cord injury research. The vigorous NINDS extramural stem cell portfolio supports research on stem cell biology in the nervous system, including the role of stem cells in the developing brain and on the application of stem cells to neurological diseases. The institute is also active in trans-NIH stem cell research efforts, including training and infrastructure programs and solicitations on cross-cutting issues, such as the development of non-embryonic sources of human pluripotent stem cells. Current research projects focus on adult and embryonic animal stem cells and on human adult and NIH-approved lines of human embryonic stem cells.
Budget Policy: The 2009 budget estimate of $154,889,000 for Repair and Plasticity represents a decrease of $170,000 from the 2008 enacted. NINDS continues to balance investigator-initiated research and solicitations. A 2009 program announcement with special review will solicit research on advanced integrated neural prosthesis research and development, reflecting the shift in emphasis from contract to grants as the neural prosthesis research field has matured. A continuing program announcement solicits research to develop advanced tools and technologies for deep brain stimulation in movement disorders.
Portrait of a Program: Neural Prosthesis Program
FY 2008 Level: $31M
FY 2009 Level: $31M
Change: $0M
Neural prostheses are devices that restore nervous system functions that are lost from disease or trauma. For 35 years, NINDS has pioneered the development of neural prostheses by engaging experts from many disciplines of engineering, materials science, and basic and clinical neuroscience, including nanotechnology. Cochlear implants that restore hearing were an early success of this program. Neural prostheses now available or in development aid breathing, hand grasp, bowel and bladder control, and cough, which are critical to quality of life for people with paralysis. Devices can enable the coordinated activation of paralyzed muscles for purposeful movement, or enable control of computers and other devices by signals directly recorded from the brain. The program has contributed to development of deep brain stimulation (DBS) therapies that dramatically help some people with essential tremor, dystonia, and Parkinson's disease. Advancing DBS technology and its potential applications to a wide variety of neurological and psychiatric disorders are now an important program focus.
In addition to its direct effects, the NINDS program spawned private sector and other government efforts. NINDS now coordinates neural prosthesis research with other components of the NIH, including the National Institute of Biomedical Imaging and Bioengineering, the National Institute on Deafness and Other Communications Disorders, and the National Center for Medical Rehabilitation, and with other parts of the government, including the Departments of Defense and Veterans Affairs. As research in this field matures, NINDS has been moving support from contract to grant mechanisms. In 2009, the Institute will issue a program announcement for grants that will support design, development, and demonstration of advanced neural prosthetics, and the institute is continuing an ongoing program announcement on tools and technologies for deep brain stimulation.
Systems and Cognitive Neuroscience
Circuits and systems of nerve cells in the brain, spinal cord, and body control learning, memory, attention, language, thinking, emotion, movement, the sleep-wake cycle, pain perception, feeding, and other complex behaviors. NINDS supports basic research on how the healthy nervous systems carries out these functions and on counteracting the disruptive effects of neurological disorders, including stroke, trauma, and neurodegenerative diseases. Neuroendocrine disorders, sleep disorders, migraine, and other chronic pain conditions are also important areas of systems and cognitive neuroscience. As the largest NIH supporter of research on pain, NINDS is a leader of the NIH Pain Consortium, which promotes collaboration among the many NIH institutes and centers that address pain. Program announcements on cognitive deficits in central nervous system disorders and on sleep and sleep disorders are continuing in 2008.
Budget Policy: The 2009 budget estimate of $192,807,000 for Systems and Cognitive Neuroscience represents a decrease of $212,000 from the 2008 enacted. NINDS continues to balance investigator initiated research and solicitations, including projects funded through the Institute's broad translational research and clinical trials programs. Based on a thorough review of NIH pain related activities, NINDS and the NIH Pain Consortium issued program announcements, which will continue into 2009, on the neurobiology of migraine, on temporomandibular joint and muscle disorders, and on mechanisms, models, measurement, and management in pain research.
Technology Development, Infrastructure, and Resources:
Standing NINDS programs foster preclinical therapeutics development, provide research resources, and support clinical trials. Although these broad programs continue from year to year, specific research projects within these programs change in response to the scientific opportunities. The Cooperative Program in Translational Research, a new medicinal chemistry support program, the SMA Project, and the Anticonvulsant Screening program are among the preclinical drug development programs. NINDS actively coordinates these preclinical efforts with NIH-wide programs, such as the Roadmap Rapid Access to Intervention Development (RAID) and the Molecular Libraries Screening Programs, in which the institute plays a leading role. Similarly, continuing NINDS clinical trials programs support research ranging from pilot studies to the planning and execution of major phase III multi-site clinical trials within all disease programs of the institute. Among recently developed clinical trials resources, the Neurological Emergencies Treatment Trials (NETT) network focuses on stroke, head and spinal cord trauma, status epilepticus (continuous seizures), and other neurological emergencies, and the Clinical Research Collaboration (CRC) engages physicians in the community to speed clinical research through enhanced recruitment to clinical trials and to minimize costs, improve access to clinical trials for diverse patients, and encourage the transfer of research results to clinical practice in community settings. Among the continuing programs of the Office of Minority and Health Research, the Specialized Neuroscience Research Programs (SNRPs) strengthen the research capabilities of basic and clinical neuroscience research programs at minority institutions. NINDS is also playing a leading role in the NIH CounterACT program, which is developing countermeasures against chemical threat agents through extensive cooperation within NIH and with the Department of Defense. This research also has significant potential for improving treatment for common neurological problems, such as seizures.
Budget Policy: The 2009 budget estimate of $64,827,000 for Technology Development, Infrastructure, and Resources represents a decrease of $72,000 from the 2008 enacted. The NINDS is renewing the Cooperative Program in Translational Research which began in 2003 and has since increased its efforts to engage small businesses, added funding for resource centers, and is now expanding to include molecular diagnostics. Currently funds projects are developing drug, stem cell, or gene therapies for more than 20 diseases, including ALS, Batten disease, epilepsy, Huntington's disease, muscular dystrophy, Parkinson's disease, tuberous sclerosis, traumatic brain injury and stroke. Among other major efforts continuing in 2009 are the SMA (spinal muscular atrophy) Project, the Anticonvulsant Screening Program, Microarray Centers, the Specialized Neuroscience Research Programs (SNRPs), medicinal chemistry support, Clinical Research Collaboration (CRC) and Neurological Emergencies Treatment Trials (NETT) clinical trials infrastructure, research cores and other technology, infrastructure, and resource programs.
Portrait of a Program: Collaborative Activities to Promote Translational Research (CAPTR)
FY 2008 Level: $1M
FY 2009 Level: $1M
Change: $0M
Translational research develops insights from basic or clinical neuroscience to therapies that are ready for testing in people. By its nature, translational research requires collaboration among investigators from multiple disciplines.
In 2007, the NINDS began a program to provide administrative supplements to current grantees to undertake new collaborations focused on translational research for neurological disorders. Although the individual awards were capped at $50,000 per grantee and $100,000 for each consortium activity, these supplements catalyzed a wide range of innovative research. The strong response reflects, at least partly, the rapid turnaround that the use of the administrative supplements allows, with about 3 months from application to award.
In its first year, the CAPTR program funded high quality proposals for a wide range of diseases, including pain, spinal muscular atrophy, traumatic brain injury, spinal cord injury, stroke, epilepsy, and migraine. Based on the strong initial response, the NINDS plans to continue program resources at a level of approximately $1 million through 2009, with possible increased funding, depending on the quality of the applications. The Institute is monitoring the success of the program, as evident through peer-reviewed publications, successful grant applications to continue work begun with these supplements, and, ultimately, the development of interventions that improve the prevention and treatment of neurological disorders.
Intramural Research
The NINDS Intramural Research Program conducts basic, translational, and clinical research on the NIH campus in Bethesda, Maryland, which is the largest community of neuroscientists in the world. Among the unique resources of the NIH campus, the Mark O. Hatfield Clinical Center is a hospital totally dedicated to clinical research and the NIH Porter Neuroscience Research Center integrates neuroscience across NIH institutes and disciplinary boundaries. Ongoing Intramural activities that respond to high institute priorities include a joint brain tumor program with the National Cancer Institute, the Suburban Hospital and Washington Hospital Center Stroke centers, pioneering research on neural stem cells, investigations of biomarkers to accelerate therapy development for multiple sclerosis, translating gene findings to therapies for neurogenetic diseases, and research on the consequences of head trauma in military personnel.
Budget Policy: The 2009 budget estimate of $147,157,000 for the Intramural Research Program represents an increase of $2,175,000 from the 2008 enacted. NINDS will add two tenure track investigators to the Neurogenetics Branch to pursue exceptional opportunities that are emerging for progress toward therapies for neurogenetic disorders, and will also expand the program in cell biology, a rapidly advancing area of research that has implications for many neurological diseases.
Research Management and Support (RMS)
NINDS RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute's programs, regulatory compliance, international coordination, and liaison with other Federal agencies, Congress, and the public.
Budget Policy: The 2009 budget estimate of $54,066,000 represents an increase of $799,000 from the 2008 enacted.
Budget Authority by Object
| FY 2008 Enacted |
FY 2009 Estimate |
|
|---|---|---|
| Total compensable workyears: | ||
| Full-time employment | 517 | 525 |
| Full-time equivalent of overtime and holiday hours | 1 | 1 |
| Average ES salary | $159,140 | $161,974 |
| Average GM/GS grade | 13.4 | 13.8 |
| Average GM/GS salary | $91,259 | $105,088 |
| Average salary, grade established by act of | ||
| July 1, 1944 (42 U.S.C. 207) | $155,402 | $159,269 |
| Average salary of ungraded positions | 110,986 | 113,459 |
| FY 2008 | FY 2009 | |
OBJECT CLASSES |
Estimate | Estimate |
| Personnel Compensation: | ||
| 11.1 Full-time permanent | $36,137,000 | $38,165,000 |
| 11.3 Other than full-time permanent | 12,144,000 | 12,820,000 |
| 11.5 Other personnel compensation | 1,392,000 | 1,470,000 |
| 11.7 Military personnel | 667,000 | 704,000 |
| 11.8 Special personnel services payments | 7,525,000 | 7,897,000 |
| Total, Personnel Compensation | 57,865,000 | 61,056,000 |
| 12.0 Personnel benefits | 13,533,000 | 14,291,000 |
| 12.2 Military personnel benefits | 426,000 | 450,000 |
| 13.0 Benefits for former personnel | 0 | 0 |
| Subtotal, Pay Costs | 71,824,000 | 75,797,000 |
21.0 Travel and transportation of persons |
3,673,000 | 3,484,000 |
| 22.0 Transportation of things | 228,000 | 216,000 |
| 23.1 Rental payments to GSA | 0 | 0 |
| 23.2 Rental payments to others | 123,000 | 123,000 |
| 23.3 Communications, utilities and miscellaneous charges | 1,005,000 | 948,000 |
| 24.0 Printing and reproduction | 404,000 | 374,000 |
| 25.1 Consulting services | 7,908,000 | 7,881,000 |
| 25.2 Other services | 9,567,000 | 9,358,000 |
| 25.3 Purchase of goods and services from government accounts | 128,109,000 | 128,761,000 |
| 25.4 Operation and maintenance of facilities | 371,000 | 353,000 |
| 25.5 Research and development contracts | 32,541,000 | 32,541,000 |
| 25.6 Medical care | 1,885,000 | 1,803,000 |
| 25.7 Operation and maintenance of equipment | 11,803,000 | 11,441,000 |
| 25.8 Subsistence and support of persons | 0 | 0 |
| 25.0 Subtotal, Other Contractual Services | 192,184,000 | 192,138,000 |
| 26.0 Supplies and materials | 8,806,000 | 8,413,000 |
| 31.0 Equipment | 5,910,000 | 5,638,000 |
| 32.0 Land and structures | 0 | 0 |
| 33.0 Investments and loans | 0 | 0 |
| 41.0 Grants, subsidies and contributions | 1,259,744,000 | 1,258,266,000 |
| 42.0 Insurance claims and indemnities | 0 | 0 |
| 43.0 Interest and dividends | 0 | 0 |
| 44.0 Refunds | 0 | 0 |
| Subtotal, Non-Pay Costs | 1,472,077,000 | 1,469,600,000 |
| Total Budget Authority by Object | 1,543,901,000 | 1,545,397,000 |
Includes F T E's which are reimbursed from the NIH Roadmap for Medical Research
Salaries and Expenses
| OBJECT CLASSES | F Y 2008 Estimate | F Y 2009 Estimate | Increase or Decrease |
|---|---|---|---|
| Personnel Compensation: | |||
| Full-time permanent (11.1) | $36,137,000 | $38,165,000 | $2,028,000 |
| Other than full-time permanent (11.3) | 12,144,000 | 12,820,000 | 676,000 |
| Other personnel compensation (11.5) | 1,392,000 | 1,470,000 | 78,000 |
| Military personnel (11.7) | 667,000 | 704,000 | 37,000 |
| Special personnel services payments (11.8) | 7,525,000 | 7,897,000 | 372,000 |
| Total Personnel Compensation (11.9) | 57,865,000 | 61,056,000 | 3,191,000 |
| Civilian personnel benefits (12.1) | 13,533,000 | 14,291,000 | 758,000 |
| Military personnel benefits (12.2) | 426,000 | 450,000 | 24,000 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 |
| Subtotal, Pay Costs | 71,824,000 | 75,797,000 | 3,973,000 |
Travel (21.0) |
3,673,000 | 3,484,000 | (189,000) |
| Transportation of things (22.0) | 228,000 | 216,000 | (12,000) |
| Rental payments to others (23.2) | 123,000 | 123,000 | 0 |
| Communications, utilities and miscellaneous charges (23.3) | 1,005,000 | 948,000 | (57,000) |
| Printing and reproduction (24.0) | 404,000 | 374,000 | (30,000) |
| Other Contractual Services: | |||
| Advisory and assistance services (25.1) | 1,092,000 | 1,065,000 | (27,000) |
| Other services (25.2) | 9,567,000 | 9,358,000 | (209,000) |
| Purchases from government accounts (25.3) | 70,380,000 | 70,590,000 | 210,000 |
| Operation and maintenance of facilities (25.4) | 371,000 | 353,000 | (18,000) |
| Operation and maintenance of equipment (25.7) | 11,803,000 | 11,441,000 | (362,000) |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | 93,213,000 | 92,807,000 | (406,000) |
| Supplies and materials (26.0) | 0 | 0 | 0 |
| Subtotal, Non-Pay Costs | 98,646,000 | 97,952,000 | (694,000) |
| Total, Administrative Costs | 170,470,000 | 173,749,000 | 3,279,000 |
Authorizing Legislation
| PHS Act/ Other Citation |
U.S. Code Citation |
2007 Amount Authorized |
F Y 2008 Enacted |
2008 Amount Authorized |
F Y 2009 Budget Estimate |
|
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinate | Indefinate | ||
| National Institute of Neurological Disorders and Stroke |
Section 402(a) | 42§281 | Indefinate | Indefinate | ||
| Total, Budget Authority | 1,543,901,000 | 1,545,397,000 |
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation 1/ |
|---|---|---|---|---|
| 2000 | 890,816,000 2/ | 979,281,000 | 1,019,271,000 | 1,034,886,000 |
| Rescission | 0 | 0 | 0 | (5,510,000) |
| 2001 | 1,050,412,000 2/ | 1,185,767,000 | 1,189,425,000 | 1,176,482,000 |
| Rescission | (383,000) | |||
| 2002 | 1,316,448,000 2/ | 1,306,321,000 | 1,352,055,000 | 1,328,188,000 |
| Rescission | (1,522,000) | |||
| 2003 | 1,432,305,000 | 1,432,305,000 | 1,466,005,000 | 1,466,005,000 |
| Rescission | (9,529,000) | |||
| 2004 | 1,468,926,000 | 1,468,326,000 | 1,510,926,000 | 1,510,776,000 |
| Rescission | (9,569,000) | |||
| 2005 | 1,545,623,000 | 1,545,623,000 | 1,569,100,000 | 1,539,448,000 |
| Rescission | (12,675,000) | |||
| 2006 | 1,550,260,000 | 1,550,260,000 | 1,591,924,000 | 1,550,260,000 |
| Rescission | (1,503,000) | |||
| 2007 | 1,524,750,000 | 1,524,750,000 | 1,537,703,000 | 1,534,757,000 |
| Rescission | (0) | |||
| 2008 | 1,537,019,000 | 1,559,106,000 | 1573268000 | 1,571,353,000 |
| Rescission | (27,452,000) | |||
| 2009 | 1,545,397,000 |
1/ Reflects enacted supplementals, rescissions, and reappropriations.
2/ Excludes funds for HIV/AIDS research activities consolidated in the NIH Office of AIDS Research.
Detail of Full-Time Equivalent Employment (FTE)
| OFFICE/DIVISION | F Y 2007 Actual | F Y 2008 Enacted | F Y 2009 Estimate |
|---|---|---|---|
| Office of the Director | 52 | 52 | 52 |
| Division of Intramural Research | 364 | 364 | 370 |
| Division of Extramural Activities | 101 | 101 | 103 |
| Total | 517 | 517 | 525 |
| Includes F T E's which are reimbursed from the NIH Roadmap for Medical Research | |||
| F T E's supported by funds from Cooperative Research and Development Agreements | (2) | (2) | (2) |
| FISCAL YEAR | Average GM/GS Grade |
|---|---|
| 2005 | 11.3 |
| 2006 | 10.7 |
| 2007 | 13.2 |
| 2008 | 13.4 |
| 2009 | 13.8 |
Detail of Positions
| GRADE | F Y 2007 Actual | F Y 2008 Enacted | F Y 2009 Estimate |
|---|---|---|---|
| Total, ES Positions | 3 | 3 | 3 |
| Total, ES Salary | 466,208 | 477,420 | 485,923 |
| GM/GS-15 | 34 | 35 | 39 |
| GM/GS-14 | 34 | 34 | 36 |
| GM/GS-13 | 73 | 73 | 72 |
| GS-12 | 58 | 58 | 59 |
| GS-11 | 46 | 46 | 45 |
| GS-10 | 5 | 6 | 5 |
| GS-9 | 33 | 34 | 35 |
| GS-8 | 19 | 19 | 20 |
| GS-7 | 14 | 14 | 15 |
| GS-6 | 2 | 1 | 2 |
| GS-5 | 0 | 1 | 0 |
| GS-4 | 0 | 2 | 2 |
| GS-3 | 1 | 0 | 0 |
| GS-2 | 1 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 320 | 323 | 330 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 2 |
| Senior Grade | 4 | 5 | 5 |
| Full Grade | 1 | 1 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 6 | 7 | 7 |
| Ungraded | 197 | 201 | 209 |
| Total permanent positions | 395 | 395 | 391 |
| Total positions, end of year | 526 | 528 | 534 |
| Total full-time equivalent (FTE) | |||
| employment, end of year | 517 | 517 | 525 |
| Average ES salary | 155,402 | 159,140 | 161,974 |
| Average GM/GS grade | 13 | 13 | 14 |
| Average GM/GS salary | 90,466 | 91,259 | 105,088 |
Includes F T E's which are reimbursed from the NIH Roadmap for Medical Research.
New Positions Requested
| FY 2009 | |||
|---|---|---|---|
| Grade | Number | Annual Salary | |
| Program Director | GS-14 | 2 | $111,104 |
| Intramural Fellow | AD | 6 | $75,000 |
| Total Requested | 8 | ||
Last updated April 18, 2008




