Publications:
Chapter 10: Pertussis
Manual for the Surveillance of Vaccine-Preventable Diseases (4th Edition, 2008)
On This Page:
![]() Print-friendly version of this page/chapter
Print-friendly version of this page/chapter ![]() (458 KB)
(458 KB)
Kristin Brown, MPH; Pam Cassiday, MS; Maria Lucia Tondella, PhD; Amanda Cohn, MD; Kris Bisgard, DVM, MPH
Disease Description
Pertussis, a cough illness commonly known as whooping cough, is caused by the bacterium Bordetella pertussis. The illness is characterized by a prolonged paroxysmal cough often accompanied by an inspiratory whoop. Disease presentation varies with age and history of previous exposure or vaccination. Young infants can present to a clinic or hospital with apnea and no other disease symptoms. Adults and adolescents with some immunity can exhibit only mild symptoms or have the typical prolonged paroxysmal cough. In all persons, cough can continue for months.
Severe disease is infrequent in healthy, vaccinated persons. Infants, particularly those who have not received a primary vaccination series, are at risk for complications and mortality. Pneumonia is the most common complication in all age groups. Seizures and encephalopathy are rare and generally only reported in young infants. Death is rare and most likely to occur in young, unvaccinated infants, although fatalities are occasionally reported among older children and adults with serious underlying health conditions.1 Pertussis can be either the cause (primary) or contributing (secondary) cause of death.
Three other Bordetella species cause disease in humans: B. parapertussis, B. holmesii, and B. bronchiseptica. B. parapertussis causes a pertussis-like illness that is generally milder than pertussis because the bacteria do not produce pertussis toxin. Co-infection of B. pertussis and B. parapertussis is not unusual. Disease attributable to Bordetella species other than B. pertussis is not reportable to CDC.
Background
In the pre-vaccine era, pertussis was a common childhood disease and a major cause of child and infant mortality in the United States. Routine childhood vaccination led to a reduction in disease incidence from an average of 150 reported cases per 100,000 persons between 1922 and 1940 to 0.5 per 100,000 in 1976.2 The incidence of reported pertussis began increasing in the 1980s, and in 2005, the incidence of reported pertussis was 8.6 per 100,000 persons (CDC, unpublished data). Reasons for this increase are not fully understood, but likely contributing factors include increased awareness of the disease and the increased use of diagnostic tests for adolescents and adults.
From 2001 through 2003, persons older than10 years of age accounted for 56% of reported cases,3 more than double the 24% they accounted for from 1990 to 1993.3, 4
Despite this increase in reported pertussis among adolescents and adults, incidence remained highest among young infants.3 In 2005, most (38 of 39) pertussis-related deaths reported to CDC were among infants aged younger than 6 months, who were too young to have received three doses of DTaP vaccine (CDC, unpublished data).
Importance of Rapid Case Identification
Early diagnosis and treatment might limit disease spread. When pertussis is strongly suspected, attempts to identify and provide prophylaxis to close contacts should proceed without waiting for laboratory confirmation. When suspicion of pertussis is low, the investigation can be delayed until there is laboratory confirmation of the diagnosis. However, prophylaxis of infants and their household contacts should not be delayed because pertussis can be severe and life-threatening to young infants.
Importance of Surveillance
Surveillance data collected through case investigation are used to assess burden of disease and guide policy and development of control strategies. CDC uses surveillance data to monitor national trends in disease and identify populations at risk. Local and state health departments use surveillance data to identify clusters of related cases that might indicate an outbreak.
Surveillance data have also been used to guide vaccination policy development. Data collected through an enhanced surveillance program suggested that infants often acquire pertussis from close contacts and supported recommendations for vaccination of postpartum mothers and adult and adolescent contacts of infants.5–7
Laboratory surveillance to monitor changes in the B. pertussis organism is also important. See Section VII, “Laboratory Testing” for more details.
Disease Reduction Goals
A disease reduction goal of 2,000 indigenous pertussis cases per year in children younger than 7 years of age was proposed as a part of the Healthy People 2010 project.8 In 2005, 7,347 cases were reported in this group (CDC, unpublished data).
Case Definition
The following case definition for pertussis was approved by the Council of State and Territorial Epidemiologists (CSTE) in June 1997.9
Clinical case definition
A cough illness lasting at least 2 weeks with one of the following: paroxysms of coughing, inspiratory “whoop,” or posttussive vomiting; and without other apparent cause (as reported by a healthcare professional).
Laboratory criteria for diagnosis
- Isolation of B. pertussis from a clinical specimen
- Positive polymerase chain reaction (PCR) assay for B. pertussis DNA
Case classification
Probable: Meets the clinical case definition, is not laboratory confirmed, and is not epidemiologically linked to a laboratory-confirmed case.
Confirmed:
- A case of acute cough illness of any duration with a positive culture for B. pertussis
- A case that meets the clinical case definition and is confirmed by PCR
- A case that meets the clinical definition and is epidemiologically linked directly to a case confirmed by either culture or PCR
Comment: The clinical case definition was designed to increase sensitivity for detecting pertussis cases when confirmatory laboratory testing was not done or was negative. Laboratory tests can be negative even when the patient has pertussis. The clinical case definition is appropriate for endemic or sporadic cases. In outbreak settings, including household exposures, a clinical case can be defined as an acute cough illness lasting 2 weeks or longer without other symptoms. A case definition of cough illness lasting 14 days or longer has demonstrated 84% sensitivity and 63% specificity for detecting culture-positive pertussis in outbreak settings.10
Collection of epidemiologic and clinical data is essential for reporting cases that meet the clinical case definition. Investigators should collect information on paroxysms of cough, whoop, and posttussive vomiting; when that is not possible, information on duration of cough should be collected for each suspected case. When feasible, case investigations initiated shortly after cough onset should include follow-up calls to collect information on cough duration. Follow-up should be done regardless of confirmatory test results so that cases meeting the clinical case definition can be reported. Both probable and confirmed pertussis cases should be reported to the National Notifiable Diseases Surveillance System (NNDSS) by the state health department via the National Electronic Telecommunications System for Surveillance (NETSS) or National Electronic Disease Surveillance System (NEDSS).
Laboratory confirmation of pertussis is important because other pathogens can cause symptoms similar to pertussis. Culture of B. pertussis is the most specific diagnostic test; all patients with cough and a positive B. pertussis culture should be reported as confirmed, even those with cough lasting less than 14 days. PCR is less specific than culture; cases confirmed with only a positive PCR must meet the clinical case definition to be reported as confirmed. To confirm a case by epidemiologic linkage, the case must be directly linked (i.e., a first-generation contact) to a laboratory-confirmed case by either culture or PCR.9
Laboratory Testing
Determining who has pertussis and who does not is often difficult. Whenever possible, a nasopharyngeal swab or aspirate should be obtained from all persons with suspected cases. A properly obtained nasopharyngeal swab or aspirate is essential for optimal results. Health department personnel who are asked to obtain these specimens should receive training and supervision from persons experienced in collection of nasopharyngeal specimens.
Culture
Isolation of B. pertussis by bacterial culture is the standard pertussis diagnostic laboratory test. A positive culture for B. pertussis confirms the diagnosis of pertussis. Culture of the organism is also necessary for antimicrobial susceptibility testing and molecular typing.
Although bacterial culture is specific for diagnosis, it is relatively insensitive. Fastidious growth requirements make B. pertussis difficult to isolate. Isolation of the organism using direct plating is most successful during the catarrhal stage (i.e., first 1–2 weeks of cough). Success in isolating the organism declines if the patient has received prior antibiotic therapy effective against B. pertussis, if specimen collection has been delayed beyond the first 2 weeks of illness, and if the patient has been vaccinated.
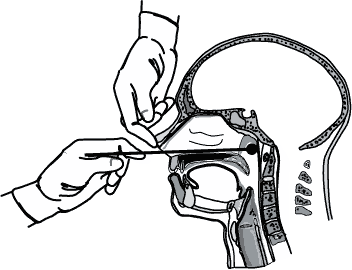
All persons with suspected cases of pertussis should have a nasopharyngeal aspirate or swab obtained from the posterior nasopharynx for culture. For B. pertussis, nasopharyngeal aspirates will yield similar or higher rates of recovery than nasopharyngeal swabs;11–14 throat and anterior nasal swabs yield unacceptably low rates of recovery.15 Therefore, specimens should be obtained from the posterior nasopharynx (Figure 1), not the throat. Specimens should be obtained using Dacron® or rayon swabs and should be plated directly onto selective culture medium or placed in transport medium. Regan-Lowe agar or freshly prepared Bordet-Gengou medium is generally used for culture; half-strength Regan-Lowe can be used as the transport medium.
Polymerase chain reaction for B. pertussis DNA
PCR testing of nasopharyngeal swabs or aspirates can be a rapid and sensitive method of diagnosing pertussis.16, 17 Since its inclusion in the case definition in 1997, the proportion of cases confirmed by PCR has increased, and many laboratories now use only PCR to confirm pertussis. As of February 2007, there are no standardized PCR assays for pertussis; assay procedures, as well as sensitivity and specificity can vary greatly between laboratories. CDC recommends that PCR be used alongside culture, rather than as an alternative test. Direct comparison with culture is necessary for validation of PCR tests performed in different laboratories. Even when a laboratory has validated its PCR method, culturing for B. pertussis should continue; this is especially important when an outbreak is suspected, because isolation of the bacterium confirms pertussis (see above). State laboratories should retain the capability to culture pertussis.
Collection methods for PCR are similar to those for culture, and often the same sample can be used for both tests. However, calcium alginate swabs cannot be used to collect nasopharyngeal specimens for PCR.
Figure 1: Proper technique for obtaining a nasopharyngeal specimen for isolation of Bordetella pertussis
Serologic testing
Although serologic testing of persons aged 11 years or older who were vaccinated 3 or more years prior to disease has been used to diagnose pertussis in clinical studies, standardized tests are not available. Some practitioners use commercial tests to diagnose pertussis, but the results of these tests are difficult to interpret. At this time, positive serology results from a private laboratory are not confirmatory for the purpose of reporting. A single-point serologic assay has been validated at the Massachusetts state public health laboratory for persons aged 11 years or older and is used for clinical diagnosis and reporting in that state only.18 A serologic test performed at CDC or at the Massachusetts state laboratory might be used to help investigate large outbreaks. Few other validated diagnostic serologic tests are available in the United States. In states other than Massachusetts, cases meeting the clinical case definition that are serologically positive but not culture or PCR positive should be reported as probable cases.
Direct fluorescent antibody testing
Direct fluorescent antibody (DFA) testing of nasopharyngeal secretions is sometimes used to screen for pertussis. While DFA testing can provide rapid results to providers treating ill infants, these results are not confirmatory because the tests are of variable specificity.13 Since it is not a confirmatory test, DFA should be used alongside culture or PCR. Cases meeting the clinical case definition that are DFA positive but not culture or PCR positive should be reported as probable cases.
Pulsed-field gel electrophoresis
Pulsed-field gel electrophoresis (PFGE), a type of DNA fingerprinting, can be performed on B. pertussis isolates to help track transmission (e.g., strains from the same household or small community), and PFGE results might show diversity within larger geographic areas such as cities, counties, and states. It is not done for routine surveillance.19, 20
Inquiries regarding PFGE molecular typing, erythromycin susceptibility testing, serologic testing and other B. pertussis laboratory questions should be directed to the CDC Epidemic Investigations Laboratory: Dr. M. Lucia Tondella, at 404-639-1239, or Ms. Pam Cassiday at 404-639-1231. When sending B. pertussis samples to CDC, please make appropriate arrangements with the laboratory before shipping samples to the address below:
Centers for Disease Control and Prevention
1600 Clifton Road, NE
DASH Unit 12
Atlanta, GA 30333
Additional information on use of the laboratory for support of vaccine-preventable disease surveillance is available in Chapter 22, “Laboratory Support for Surveillance of Vaccine-Preventable Diseases.”
Reporting
Each state and territory has regulations or laws governing the reporting of diseases and conditions of public health importance.21 These regulations and laws list the diseases to be reported and describe those persons or institutions responsible for reporting, including healthcare providers, hospitals, laboratories, schools, daycare and childcare facilities, and other institutions. Persons reporting should contact the state health department for state-specific reporting requirements.
Reporting to CDC
State health departments should report all probable and confirmed pertussis cases to NNDSS via the NETSS or NEDSS. When provisional information is reported to NNDSS, NETSS and NEDSS reports can be updated as additional information is collected. NETSS and NEDSS accept information about clinical symptoms, laboratory confirmation and vaccination history; this information is included in the Pertussis Surveillance Worksheet (Appendix 11) available for reference and use in case investigation.
CDC recommends investigation of deaths associated with B. pertussis, regardless of whether they meet the CSTE pertussis case definition. Investigators are requested to complete the Pertussis Death Report Worksheet (Appendix 12) and forward the worksheet and copies of the documents listed therein to CDC at the direction of the state health department.
Information to collect
Case investigation should include collection of the epidemiologic information listed below. State health departments often supplement this list with additional information relevant to cases in their communities.
- Demographic
- Name
- State of residence
- Date of birth
- Age
- Sex
- Ethnicity
- Race
- Reporting Source
- County
- Earliest date reported
- Clinical
- Hospitalization and duration of stay
- Cough, date of cough onset and duration
- Symptoms: paroxysms, whoop, posttussive vomiting, apnea
- Complications: pneumonia (x-ray results), seizures, encephalopathy
- Outcome (patient survived or died) and date of death
- Treatment
- Antibiotics used
- Date started and duration of therapy
- Laboratory
- Culture
- PCR
- Serology for antibody to pertussis antigens
- Vaccination with pertussis-containing vaccine
- Dates of vaccination
- Type (formulation) of vaccines and manufacturers’ names
- Doses of pertussis-containing vaccine prior to illness onset
- If not vaccinated with three doses, reason
- Epidemiologic
- Date case investigation initiated
- Epidemiologic linkage to a laboratory-confirmed case
- Association with an outbreak
- Transmission setting
- Setting outside household of further documented spread
Comments on reporting
The limitations of laboratory diagnostics make the clinical case definition essential to pertussis surveillance. It is important to determine duration of cough—specifically whether it lasts 14 days or longer—in order to determine if a person’s illness meets the definition of a clinical case. If the first interview is conducted within 14 days of cough onset and cough is still present at the time of interview, it is important to follow up at 14 days or later after onset.
Accurate assessment of pertussis symptoms can be challenging. The following symptom definitions and variable explanations are appropriate for pertussis case investigations.
Paroxysmal or spasmodic cough. Sudden uncontrollable “fits” or spells of coughing where one cough follows the next without a break for breath.
Whoop. High-pitched noise heard when breathing in after a coughing spasm.
Apnea. Transient cessation of respiration which might occur spontaneously or after a coughing spasm. Apnea is generally associated with cyanosis or syncope (passing out) and might be accompanied by slowing of the heartbeat (bradycardia). Apnea is a common pertussis symptom in infants and might be the only presenting sign of pertussis in young infants with no cough; apnea is rarely associated with pertussis in older children and adults.
Cyanosis. Paleness or blueness of the skin, most noticeable on the lips and tongue, occurring after coughing paroxysms and apnea.
Posttussive vomiting. Vomiting following paroxysms of cough.
Cold-like symptoms. Coryza (runny nose) and/or conjunctival infection (redness of the eyes).
Positive chest x-ray for pneumonia. Evidence of acute pneumonia on chest x-ray.
Acute encephalopathy. Acute illness of the brain manifested by a decreased level of consciousness (excluding transient drowsiness after a seizure) occurring with or without seizures. Patients are almost always hospitalized and most undergo extensive diagnostic evaluations.
Vaccination
Currently, the pertussis vaccines available in the United States are acellular pertussis antigens in combination with diphtheria and tetanus toxoids (DTaP, DTaP, combination vaccines, and Tdap).
The Advisory Committee on Immunization Practices (ACIP) recommends a four-dose primary series of DTaP, administered at 2, 4, 6 and 15–18 months of age, followed by a fifth booster dose given at 4–6 years.21 In 2005 and 2006, the ACIP recommended the replacement of a single Td booster with a dose of Tdap for adolescents (ages 11–18) and adults (ages 19–64)6, 22 who have not previously received Tdap.
Table 1 lists vaccines likely to appear in case-patients’ vaccination histories. Immunization registries, provider records, and parents are the best sources of this information.
Table 1. Persussis-containing vaccines |
||
Pertussis-Containing Vaccines for Children |
Brand |
Licensed Date and Used For |
|---|---|---|
| DTaP | INFANRIX® DAPTACEL® Tripedia® |
First licensed in 1991; used for all childhood doses |
| DTaP+Hib | TriHiBit® | Used for the fourth dose only |
| DTap+IPV+HepB | PEDIARIX® | Used for the first three doses |
| DTap+IPV+Hib | PENTACEL™ | Approved in 2008; used for primary four-dose series |
| DTap+IPV | KINRIX™ | Approved in 2008; used for booster dose at 4-6 years |
Pertussis-Containing Vaccines for Adolescents and Adults |
Brand |
Licensed Date |
| Tdap | ADACEL® BOOSTRIX® |
First available in 2005 |
Other Vaccines |
Brand |
Licensed Date |
| Pertussis Only | Not available in the U.S. | |
| DT/Td | DECAVAC™ TENIVAC™ |
Do not contain pertussis; DT used for primary series when pertussis vaccination was not desired; Td used in persons aged ≥7 years |
Enhancing Surveillance
A number of surveillance activities can improve detection and reporting of cases as well as the completeness and accuracy of the information reported. In addition to those outlined below, Chapter 19, “Enhancing Surveillance,” lists activities that might be applicable to pertussis surveillance.
Assuring appropriate diagnostic testing for pertussis is being performed regularly
Unlike many other vaccine-preventable diseases of childhood, pertussis remains endemic in the United States. Cases are expected to occur in all communities; a period of several years in which no cases are reported from a jurisdiction likely reflects failures to diagnose and/or report disease rather than an absence of disease. The level of diagnostic testing being undertaken can be evaluated by reviewing the number of pertussis diagnostic tests (e.g., cultures) submitted by a jurisdiction.
Monitoring surveillance indicators
Regular monitoring of surveillance indicators might identify specific areas of the surveillance and reporting system that need improvement. Some indicators are markers of the thoroughness of investigation and the timeliness of reporting:
- The proportion of probable cases that did not meet the clinical case definition because the cough duration was less than 14 days and the patient was coughing at follow-up. These are cases for which later follow-up calls can improve case status classification.
- The proportion of probable and confirmed cases with complete information on vaccination history (dates, vaccine types and manufacturers). Now that pertussis vaccination is available for adolescents and adults, many states will for the first time be collecting vaccination histories for adolescents and adults. Some electronic reporting systems will require coding changes to allow this information to be entered.
- Median interval between onset of cough and notification of state or local public health authorities in probable and confirmed cases.
Case Investigation
Case investigations generally include reviews of laboratory, hospital, and clinic records, which are the best sources for information about diagnoses and immunization histories. Investigations also include interviews of case-patients, which are necessary to identify sources of infections and contacts at risk. Investigations can include treatment of case-patients and chemoprophylaxis and or vaccination of contacts.
Treatment and chemoprophylaxis
Antimicrobial treatment does not generally lessen the severity of disease unless it is begun in the catarrhal phase, prior to paroxysmal coughing.23 Treatment reduces transmission and is essential for disease control. The spread of pertussis can be limited by decreasing the infectivity of the patient and by protecting close contacts.24 Persons with pertussis are infectious from the beginning of the catarrhal stage through the third week after the onset of paroxysms or until 5 days after the start of effective antimicrobial treatment. The recommended antimicrobial agents and doses are the same for treatment and chemoprophylaxis.25
Three macrolides are recommended by CDC for treatment of pertussis. Azithromycin is most popular because it is given in a short, simple regime of one dose each day for 5 days. It is the preferred antimicrobial for use in infants younger than 1 month of age. Similarly, the regime of two doses a day for 7 days makes clarithromycin another well-accepted choice. Erythromycin, which is given as four doses each day for 14 days, continues to be used, but adherence to the regime and completion of the course are generally lower than for the other macrolides. Resistance of B. pertussis to macrolides is rare, and antimicrobial susceptibility testing is not routinely recommended. Testing is appropriate in some circumstances and is recommended when treatment failure is suspected. Refer to Section VII, “Laboratory Testing” for information on how to contact the CDC Pertussis Laboratory to discuss susceptibility testing. If resistance to macrolides is suspected or if their use is contraindicated, CDC recommends treatment with trimethoprim–sulfamethoxazole (TMP-SMZ) in a regime of two doses a day for 14 days. TMP-SMZ should not be used to treat infants younger than 2 months of age.25
CDC recommends administration of chemoprophylaxis to all close contacts and all household members of a pertussis case-patient, regardless of age and vaccination status; this might prevent or minimize transmission. A close contact is anyone who had face-to-face contact or shared a confined space for a prolonged period of time with an infected person or had direct contact with respiratory secretions from a symptomatic person. Contact with respiratory secretions can occur in many ways, including through an explosive cough or sneeze in the face, sharing food or eating utensils, mouth-to-mouth resuscitation, and conducting a medical exam which includes nose and throat examination.25
Prophylaxis of infant contacts of persons with B. parapertussis infection should be considered, and infants with B. parapertussis infection should be treated. The same antibiotics that are used for treatment and prophylaxis of pertussis are effective for the treatment of parapertussis.25
Vaccination
Close contacts younger than 7 years of age who have not received four doses of a pertussis vaccine should complete the series using the minimum recommended intervals between doses (minimum age for first dose is 6 weeks; minimum intervals from dose 1 to dose 2, and from dose 2 to dose 3 are 4 weeks; minimum interval from dose 3 to dose 4 is 6 months). Vaccination with a fifth dose of DTaP is recommended for close contacts aged 4–6 years who have only received four doses. Adult and adolescent close contacts can be vaccinated with Tdap in accordance with ACIP recommendations. Vaccination is not a substitute for chemoprophylaxis and might not prevent illness in a person who has already been infected with B. pertussis.22, 26
Outbreak Control
Pertussis outbreaks can be difficult to identify and manage. Other respiratory pathogens often cause clinical symptoms similar to pertussis, and co-circulation with other pathogens does occur. In order to respond appropriately (e.g., provide appropriate prophylaxis), it is important to confirm that B. pertussis is circulating in the outbreak setting and to determine whether other pathogens are contributing to the outbreak. PCR tests vary in specificity, so obtaining culture confirmation of pertussis for at least one suspicious case is recommended any time there is suspicion of a pertussis outbreak.
If cases are occurring among young infants, consideration can be given to vaccinating infants at an accelerated schedule. The first dose of DTaP can be given as early as 6 weeks of age, with a minimum interval of 4 weeks between each of the first three doses. Adults in close contact with infants should always be encouraged to be vaccinated with Tdap, particularly during an outbreak. The ACIP recommends vaccination of postpartum mothers who have not previously received Tdap.6
Institutional outbreaks of pertussis are common. Outbreaks at middle and high schools can occur as protection from childhood vaccines wanes.22 In school outbreaks, prophylaxis is recommended for close classroom and team contacts. Pertussis outbreaks in hospitals and other clinical settings can put infants and other patients at risk. Healthcare personnel with pertussis and exposed healthcare personnel who are symptomatic should be relieved from direct patient contact during the infectious period or until they have completed 5 days of treatment.26 The efficacy of Tdap vaccination in controlling school or institutional outbreaks has not been evaluated; adolescents and adults who have not previously received Tdap can be vaccinated in accordance with the ACIP guidelines for Tdap use in outbreaks and settings of increased risk.22, 26
References
- Vitek CR, Pascual FB, Baughman AL, Murphy TV. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr Infect Dis J 2003;22:628–34.
- Davis SF, Strebel PM, Cochi SL, Zell ER, Hadler SC. Pertussis surveillance—United States, 1989–1991. MMWR 1992;41(No. SS-8):11–19.
- CDC. Pertussis—United States, 2001–2003. MMWR 2005;54(50):1283–6.
- Guris D, Strebel PM, Bardenheier B, Brennan M, Tachdjian R, Finch E, et al. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990–1996. Clin Infect Dis 1999; 28:1230–7.
- Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, et al. Infant pertussis: who was the source? Pediatr Infect Dis J 2004; 23:985–9.
- CDC. Prevention of diphtheria, tetanus, and pertussis among pregnant women: provisional recommendations for use of Tdap in pregnant women. Atlanta, GA. 2006. Available at http://www.cdc.gov/vaccines/recs/provisional/
- CDC. ACIP votes to recommend use of combined tetanus, diphtheria and pertussis (Tdap) vaccine for adults. 2006. Atlanta, GA: CDC, 2006.
- U.S. Department of Health and Human Services. Healthy People 2010. 2nd. ed. With understanding and improving health and objectives for improving health (2 vols.). Washington DC: US Department of Health and Human Services, 2000.
- Council of State and Territorial Epidemiologists. CSTE Position Statement 1997-ID-9: Public health surveillance, control and prevention of pertussis. CSTE, 1997. Available at http://www.cste.org/ps/1997/1997-id-09.htm (exit site)
- Patriarca PA, Biellik RJ, Sanden G, Burstyn DG, Mitchell PD, Silverman PR, et al. Sensitivity and specificity of clinical case definitions for pertussis. Am J Public Health 1988; 78:833–6.
- Bejuk D, Begovac J, Bace A, Kuzmanovic-Sterk N, Aleraj B. Culture of Bordetella pertussis from three upper respiratory tract specimens. Pediatr Infect Dis J 1995;14:64–5.
- Hallander HO, Reizenstein E, Renemar B, Rasmuson G, Mardin L, Olin P. Comparison of nasopharyngeal aspirates with swabs for culture of Bordetella pertussis. J Clin Microbiol 1993;31:50–2.
- Halperin SA, Bortolussi R, Wort AJ. Evaluation of culture, immunofluorescence, and serology for the diagnosis of pertussis. J Clin Microbiol 1989; 7:752–7.
- Hoppe JE, Weiss A. Recovery of Bordetella pertussis from four kinds of swabs. Eur J Clin Microbiol 1987;6:203–5.
- Loeffelholz M. Bordetella. In: Murray P, Barron E, Jorgenses J, Pfaller M, Yolken R, eds. Manual of clinical microbiology. Washington D.C.: American Society for Microbiology, 2003.
- Koidl C, Bozic M, Burmeister A, Hess M, Marth E, Kessler HH. Detection and differentiation of Bordetella spp. by real-time PCR. J Clin Microbiol 2007;45:347–50.
- Qin X, Galanakis E, Martin ET, Englund JA. Multi-target polymerase chain reaction for diagnosis of pertussis and its clinical implications. J Clin Microbiol 2007;45:506–11.
- Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Bicchieri R, et al. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis 1994;169:1297–1305.
- Bisgard KM, Christie CD, Reising SF, Sanden GM, Cassiday PK, Gomersall C, et al. Molecular epidemiology of Bordetella pertussis by pulsed-field gel electrophoresis profile: Cincinnati, 1989–1996. J Infect Dis 2001;183:1360–7.
- de Moissac YR, Ronald SL, Peppler MS. Use of pulsed-field gel electrophoresis for epidemiological study of Bordetella pertussis in a whooping cough outbreak. J Clin Microbiol 1994;32:398–402.
- CDC. Pertussis vaccination: Use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-7):1–25.
- CDC. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2006;559(No. RR-3):1–34.
- Edwards KM, Decker MD. Pertussis vaccine. In: Plotkin S, Orenstein WA, eds. Vaccines. Philadelphia, PA.: Saunders Co., 2004: 471–528.
- American Academy of Pediatrics. Pertussis. In: Pickering LK, ed. Red Book: 2003 Report of the Committee on Infectious Diseases. Elk Grove Village, Il.: American Academy of Pediatrics, 2003: 472–86.
- CDC. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR 2005;54(No. RR-14):1–16.
- CDC. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR 2006;55(No. RR-17):1–37.
* Due to the large size of this file, please be patient while the file is being downloaded. If the file does not download after a few minutes, close all open applications and reboot your computer before trying again.
Non-CDC Link Disclaimer: Links to non-Federal organizations found at this site are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. The CDC is not responsible for the content of the individual organization web pages found at these links.
.pdf files: To view and print the .pdf files on this site, you will need Adobe Acrobat Reader. Use this link to obtain a free copy of Adobe Acrobat Reader (exit). We highly recommend that you upgrade to the latest version if haven't already.
Content last reviewed on August 20, 2008
Content Source: National Center for Immunization and Respiratory Diseases