|
 |
 |
 |
 | |||||
| |||||||||
|
|
|
|
|
Genomics
at FDA Decision Tree also available as PDF
Submission of Pharmacogenomics Data to an IND
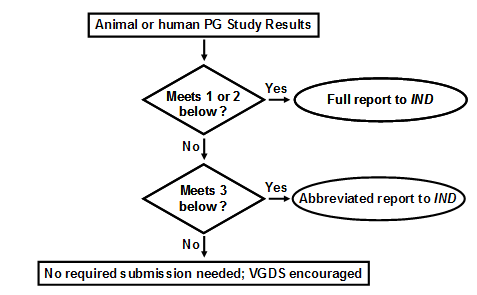
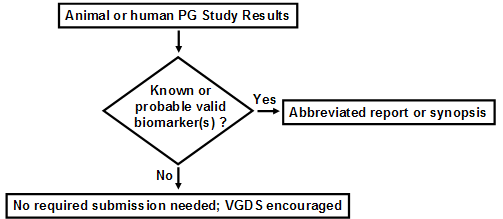
Pharmacogenomic data must be submitted to the IND under CFR 312.23 if ANY of the following apply: 1. The test results are used for making decisions pertaining to a specific clinical trial, or in a animal trial used to support safety (e.g., the results will affect dose selection, entry criteria into a clinical trial safety monitoring, or subject stratification). 2. A sponsor is using the test results to support scientific arguments pertaining to, for example, the pharmacologic mechanism of action, the selection of drug dosing, or the safety and effectiveness of a drug. 3. The test results constitute a known, or probable, valid biomarker for physiologic, pathophysiologic, pharmacologic, toxicologic, or clinical states or outcomes in humans, or is a known valid biomarker for a safety outcome in animal studies. If the information on the biomarker (example, human CYP2D6 status) is not being used for purposes 1 or 2 above, the information can be submitted to the IND as an abbreviated report. Submission to an IND is NOT needed, but voluntary submission is encouraged (i.e., information does not meet the criteria of CFR 312.23) if: 4. Information is from exploratory studies or is research data, such as from general gene expression analyses in cells/animals/humans, or single-nucleotide polymorphism (SNP) analysis of trial participants. 5. Information consists of results from test systems where the validity of the biomarker is not established. Submission of Pharmacogenomics Data to a New NDA, BLA, or Supplement
1. The sponsor will use the test results in the drug label or as part of the scientific database being used to support approval as complete submissions (not in the form of an abbreviated report, synopsis, or VGDS), including information about test procedures and complete data, in the relevant sections of the NDA or BLA. If the pharmacogenomic test is already approved by the FDA or is the subject of an application filed with the Agency, information on the test itself can be provided by cross reference.
The following examples would fit
this category:
2. The test results are known valid biomarkers for
physiologic, pathophysiologic, pharmacologic, toxicologic, or
clinical states or outcomes in the relevant species, but the
sponsor is not relying on or mentioning this in the label.
Submit to the Agency as an abbreviated report (not as a
synopsis or VGDS). If a
pharmacogenomic test of this type was conducted as part of a
larger overall study, the reporting of the pharmacogenomic test
results can be incorporated into the larger study report.
3.
3. The test results represent probable valid biomarkers for physiologic, pathophysiologic, pharmacologic, toxicologic, or clinical states or outcomes in the relevant species. Submit to the Agency as an abbreviated report. If pharmacogenomic testing of this type was conducted as part of a larger study, the abbreviated report can be appended to the report of the overall study.
4.Information from general exploratory or research studies,
such as broad gene expression screening, collection of sera or
tissue samples, or results of pharmacogenomic tests that are not
known or probable valid biomarkers to the NDA or BLA are not
required to be submitted.
Because the Agency does not view these studies as germane
in determining the safety or effectiveness of a drug, the
submission requirements in
CFR 314.50 or
601.2 will be satisfied
by the submission of a synopsis of the study.
However, the Agency encourages the voluntary submission
of the data from the study in a VGDS submitted to the NDA or BLA.
Submission of Pharmacogenomics Data to an Approved NDA, BLA, or Supplement
Date created: March 22, 2005 |