 The principal display panel, or PDP, is that portion of the package label that
is most likely to be seen by the consumer at the time of purchase. Many
containers are designed with two or more different surfaces that are suitable
for display as the PDP. These are alternate principal display panels.
The principal display panel, or PDP, is that portion of the package label that
is most likely to be seen by the consumer at the time of purchase. Many
containers are designed with two or more different surfaces that are suitable
for display as the PDP. These are alternate principal display panels.21 CFR 101.1
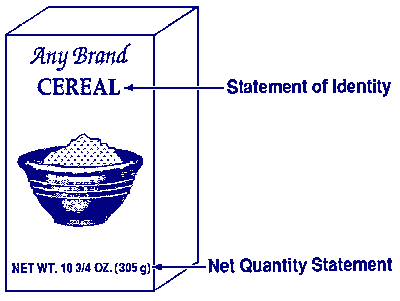 Place the statement of identity, or name of the food, and the net quantity
statement, or amount of product, on the PDP and on the alternate PDP. The
required type size and prominence are discussed in
Chapters 2 and 3.
Place the statement of identity, or name of the food, and the net quantity
statement, or amount of product, on the PDP and on the alternate PDP. The
required type size and prominence are discussed in
Chapters 2 and 3.21 CFR 101.3(a) and 101.105(a)
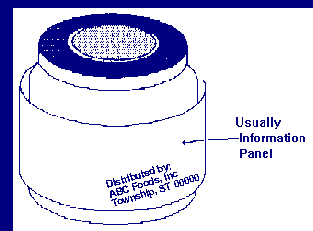
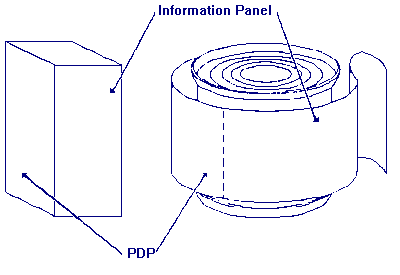 The information panel is the label panel immediately to the right
of the PDP,
as displayed to the consumer. If this panel is not usable, due to package
design and construction, (e.g., folded flaps), then the information panel is
the next label panel immediately to the right.
The information panel is the label panel immediately to the right
of the PDP,
as displayed to the consumer. If this panel is not usable, due to package
design and construction, (e.g., folded flaps), then the information panel is
the next label panel immediately to the right.21 CFR 101.2(a)
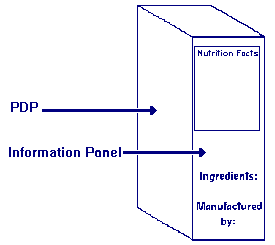 The phrase "information panel labeling" refers to the label statements that
are generally required to be placed together, without any intervening
material, on the information panel, if such labeling does not appear on the
PDP. These label statements include the name and address of the manufacturer,
packer or distributor, the ingredient list, and nutrition labeling.
The phrase "information panel labeling" refers to the label statements that
are generally required to be placed together, without any intervening
material, on the information panel, if such labeling does not appear on the
PDP. These label statements include the name and address of the manufacturer,
packer or distributor, the ingredient list, and nutrition labeling.21 CFR 101.2(b) and (d)
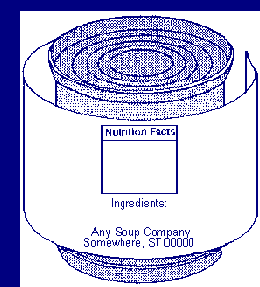 For information panel labeling, use a print or type size that is prominent,
conspicuous and easy to read. Use letters that are at least one-sixteenth
(1/16) inch in height based on the lower case letter "o". The letters must not
be more than three times as high as they are wide, and the lettering must
contrast sufficiently with the background so as to be easy to read. Do not
crowd required labeling with artwork or non-required labeling.
For information panel labeling, use a print or type size that is prominent,
conspicuous and easy to read. Use letters that are at least one-sixteenth
(1/16) inch in height based on the lower case letter "o". The letters must not
be more than three times as high as they are wide, and the lettering must
contrast sufficiently with the background so as to be easy to read. Do not
crowd required labeling with artwork or non-required labeling. Smaller type sizes may be used for information panel labeling on very small food packages as discussed in 21 CFR 101.2(c).
Different type sizes are specified for the nutrition facts label.
The type size requirements for the statement of identity and the net quantity statement are discussed in Chapters 2 and 3 of this booklet.
21 CFR 101.2(c) and 101.9(d)(1)(iii)
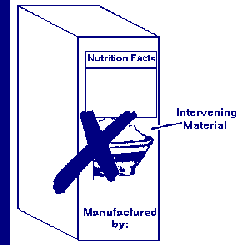 Nonessential, intervening material is not permitted to be placed between the
required labeling on the information panel (e.g., the UPC bar code is not
required labeling).
Nonessential, intervening material is not permitted to be placed between the
required labeling on the information panel (e.g., the UPC bar code is not
required labeling).21 CFR 101.2(e)
- Name and address of the manufacturer, packer or distributor. Unless
the name given is the actual manufacturer, it must be accompanied by a
qualifying phrase which states the firm's relation to the product,
e.g., "manufactured for" or "distributed by."
- Street address if the firm name and address are not listed in a
current city directory or telephone book;
- City or town;
- State (or country, if outside the United States); and
- ZIP code (or mailing code used in countries other than the United States).
21 CFR 101.5