CBER Presentation
Printable Version (PDF - 263 KB)
Common Challenges in Development of Cell and Gene Therapy Products
Well Characterized Biotechnology Pharmaceutical (WCBP) Symposium
1/28-29/2007
Mike Havert, Ph.D.
mike.havert@fda.hhs.gov
US Food and Drug Administration
Center for Biologics Evaluation and Research
Office of Cellular, Tissue, and Gene Therapies
Overview
- Types of cell and gene therapy products
- Regulatory expectations for product characterization and consistency
- New analytical technology for characterizing emerging therapeutics
Products Regulated in OCTGT
- Somatic cell therapies
- Tumor Vaccines
- Gene therapies
- Xenotransplantation
- Combination products
- Devices used for cell/tissues
- Unique assisted reproduction (ooplasm transfer)
- Anti-idiotype antibodies
- Tissue and tissue based products
Gene therapy products designed for.
- Inherited disease
- Acquired disease
- Cancer
- cytokines, immune modulators, tumor suppressor genes, suicide genes, oncolytic viruses
- Wound healing / new vasculature / improve cell survival or function
- growth factors, extracellular matrix, cell cycle regulators, signal transduction or anti-apoptotic molecules
- Chronic infection
- antisense or siRNA targeting virus
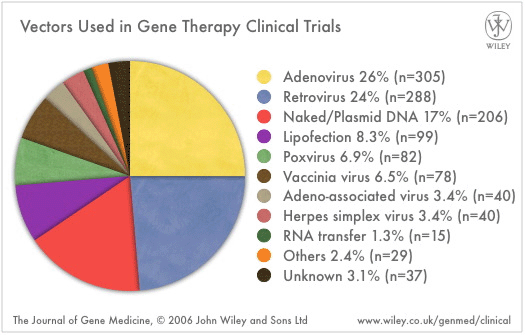
Vectors for delivery
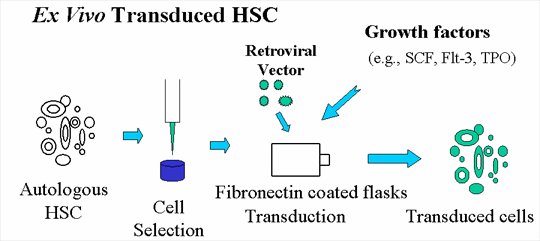
Autologous cells transduced with vector
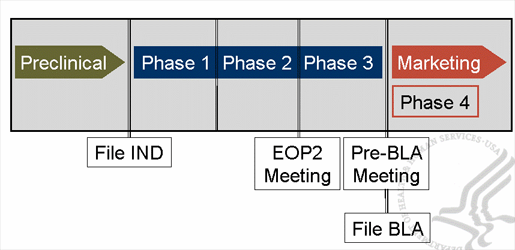
Regulatory timeline
How does a manufacturer know what test to use for their product?
- Sponsor is free to use any scientifically valid test for in-process testing of product
- For final product testing, if test method is not specified by biological product standards, sponsor can use any scientifically valid test (21 CFR 610)
- It is also possible to use alternative tests to those prescribed by biological product standards
- 21 CFR 610.9
- Information on generally acceptable types of testing:
- FDA Guidance documents
- ICH Guidance documents
- United States Pharmacopeia (USP)
- Scientific literature, etc..
General Biological Products Standards
| CFR | Test |
| 610.9 | Alternative Methods |
| 610.10 | Potency |
| 610.11 | *General Safety |
| 610.12 | Sterility |
| 610.13 | Purity |
| 610.14 | Identity |
| 610.15 | Constituent Materials |
| 610.30 | **Mycoplasma |
| 610.40 | Communicable diseases |
*Cellular Therapies are exempt
**Only required for cells that are cultured
Biologics Standards
| Required Test | Test Method | Test Timing | Specification |
| Sterility | Specified | Final Product | Negative |
| Mycoplasma | Specified | Final Product | Negative |
| Purity (pyrogenicity) | Specified | Final Product | Pass |
| Identity | Not Specified | Final Product | Product Specific |
| Potency | Not specified | Final Product | Product Specific |
| Other tests | Viability, Phenotype, etc. | Ensure safety and consistency |
Identity
- Verification that vial contents match the label
- Develop identity assay specific for the product
- Multiple active components in product?
- The test methods should identify all of them
- Distinguish the final product from other products made in the same facility
Potency Assay Wish List
- Available for release
- Consistent/validatable
- Demonstrate product activity
- Quantitative
- Stability indicating
- Demonstrate product consistency
Preparing for pivotal studies
Common challenges for Pivotal Studies
- Product characterization
- Lack of understanding of the product
- Inability to ensure product consistency
- Potency
- Assay(s) is insufficient to determine biological activity
- Assay(s) is not quantitative
- Acceptance criteria are inadequate
Solution lies in preparation
- Determine critical product characteristics and how they will be controlled
- Identify and characterize therapeutic and inactive components
- Identify and measure impurities and inactive components
- Establish a meaningful potency assay
- Refine procedures and acceptance criteria based on development experience
- Make plans for potential comparability studies for new sites and unexpected process changes
Identifying critical attributes may involve new technology
- New Technologies
- Microarray, proteomics and others
- May be useful for
- Product development
- Characterize complex products
- Identify markers predictive of behavior
- Lot release
- Potency
- Identity
Contact Information
Cell and Gene therapy product manufacturing questions
Mike Havert
mike.havert@fda.hhs.gov
301-827-5102
General CBER Issues
Office of Communication, Training & Manufacturers Assistance
Manufacturers Assistance and Technical Training Branch
Telephone: 800-835-4709 or 301-827-1800 E-mail: matt@cber.fda.gov
Internet: http://www.fda.gov/cber/manufacturer.htm


