Fatalities Reported to FDA
Following Blood Collection and Transfusion
Annual Summary for Fiscal Years 2005 and 2006
Printable version of report (PDF, 166 KB)
Note: Documents in PDF format require the Adobe Acrobat Reader®. If you experience problems with PDF documents, please download the latest version of the Reader®.
- Background
- Email us at fatalities2@fda.hhs.gov,
- Call us at 301-827-6220, or
- Write us at:
FDA/Center for Biologics Evaluation and Research
Office of Compliance and Biologics Quality
Division of Inspections and Surveillance (HFM-650)
1401 Rockville Pike, Suite 200 North
Rockville, Maryland 20852-1448 - Results
- Overall Comparison of Transfusion-Related Fatalities Reported in FY2005 and FY2006
- Transfusion Related Acute Lung Injury (TRALI)
- Hemolytic Transfusion Reactions:
- 2 cases: Recipient identification errors at the time of transfusion preceded the ABO-incompatible transfusions.
- 2 cases: Blood bank clerical errors led to ABO-incompatible transfusions.
- 1 case: Both a blood bank clerical error and an error in recipient identification at the time of transfusion preceded the incompatible transfusion.
- 1 case: Transfusion of high titer anti-A antibodies in a unit of group O Apheresis Platelets to a group A patient.14 , 15
- Microbial Infection
- Not Transfusion Related
- Transfusion Not Ruled Out as Cause of Fatality
- Post-Donation Fatalities
The blood supply is safer today than at any time in history. While the blood supply is very safe, current technology cannot entirely eliminate potential risks associated with blood transfusion. Over time, risks have decreased as a result of advances in donor screening, improved viral marker tests, automated data systems, and changes in transfusion medicine practices. The historic risks of transfusion including transmission of hepatitis, HIV, and bacteria have been greatly reduced. As some risks have diminished, other issues are receiving additional attention, including hemolytic transfusion reactions, transfusion related acute lung injury (TRALI), and transfusion associated circulatory overload (TACO). The number of transfusion related fatalities reported to the FDA is extremely small in comparison to the total number of transfusions – approximately 29 million components, of which 14.2 million were Whole Blood or Red Blood Cells (2004 calendar year).1 CBER is distributing this summary of transfusion fatality reports received by the FDA to contribute to the body of knowledge quantifying and trending the overall risks of blood transfusion. We also include information on the infrequent reports of post-donation fatalities.
Section 606.170(b) of Title 21, Code of Federal Regulations (21 CFR 606.170(b)), requires that facilities notify the Food and Drug Administration (FDA), Center for Biologics Evaluation and Research (CBER), Office of Compliance and Biologics Quality (OCBQ), as soon as possible after confirming a complication of blood collection or transfusion to be fatal. The collecting facility is to report donor fatalities, and the compatibility testing facility is to report recipient fatalities. The regulation also requires the reporting facility to submit a report of the investigation within 7 days after the fatality. In addition, 21 CFR 640.73 requires notification by telephone as soon as possible if a Source Plasma donor has a fatal reaction which, in any way, may be associated with plasmapheresis. For further information, see our Guidance for Industry: Notifying FDA of Fatalities Related to Blood Collection or Transfusion, September 2003.2
There are multiple options for providing initial notification to FDA, including email, facsimile and telephone. If facilities submit notifications by email, our email system returns an email confirmation receipt to the facility. However, if email security is a concern, facilities may not wish to use this method. See our web page, Notification Process for Transfusion Related Fatalities and Donation Related Deaths, http://www.fda.gov/cber/transfusion.htm, for more information.
A team of CBER medical officers reviews the documentation submitted by the reporting facility and obtained by the FDA investigators, to assess the relationship, if any, between the blood donation or transfusion and the reported fatality. When applicable, the review team also verifies whether the facility has implemented suitable remedial or corrective actions.
If you have questions concerning this summary, you may contact us using any of the three following options.
During Fiscal Year 2005 (FY2005) (October 1, 2004, through September 30, 2005), we received a total of 105 fatality reports. Of these reports, 97 were transfusion recipient fatalities and 8 were post-donation fatalities. Of the 97 transfusion recipient fatality reports, we concluded: a) 21 of the fatalities were unrelated to the transfusion, b) 62 of the fatalities were transfusion-related, and c) we were unable to rule out transfusion as the cause of the fatality in 14 cases.
During FY2006 (October 1, 2005, through September 30, 2006), we received a total of 95 fatality reports. Of these reports, 81 were transfusion recipient fatalities and 14 were post-donation fatalities. Of the 81 transfusion recipient fatality reports, we concluded: a) 8 of the fatalities were unrelated to the transfusion, b) 63 of the fatalities were transfusion-related, and c) we were unable to rule out transfusion as the cause of the fatality in 10 cases.
We summarize the results of our review in the following sections. Sections A through D of this document present the transfusion-related fatalities (62 in FY2005 and 63 in FY2006). Sections E and F and in Table 4 of this document present the fatalities which were not related to the transfusion, or in which the transfusion could not be ruled out as the cause of death. Section G presents the post-donation fatality reports.
A. Overall comparison of transfusion-related fatalities reported in FY2005 and FY2006
B. Transfusion Related Acute Lung Injury (TRALI)
C. Hemolytic transfusion reactions
D. Microbial infection
E. Not transfusion related
F. Transfusion not ruled out as cause of fatality
G. Post-donation fatalities
In combined FY2005 and FY2006, the highest numbers of reported fatalities were related to Transfusion Related Acute Lung Injury (TRALI), followed by non-ABO hemolytic transfusion reactions (HTR). Complications of microbial infection, ABO hemolytic transfusion reactions, and Transfusion Associated Circulatory Overload (TACO) accounted for the smallest number of reported fatalities (Table 1).
Table 1: Transfusion-Related Fatalities Reported in FY2005 and FY2006
| Complication | FY05 | FY06 | Total (FY05+FY06) | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| TRALI | 29 | 47% | 35 | 56% | 64 | 51% |
| HTR (non-ABO) | 16 | 26% | 9 | 14% | 25 | 20% |
| Microbial Infection | 8 | 13% | 7 | 11% | 15 | 12% |
| HTR (ABO) | 6 | 10% | 3 | 5% | 9 | 7% |
| TACO | 1 | 2% | 8 | 13% | 9 | 7% |
| Other* | 2 | 3% | 1 | 2% | 3 | 2% |
| Totals | 62 | 100% | 63 | 100% | 125 | 100% |
*Other:
FY2005: Includes one case of Graft vs. Host Disease (GVHD) and one therapeutic plasma exchange (TPE) error (use of a treatment column contraindicated due to patient’s medical history)
FY2006: One case of anaphylaxis (patient had high titer anti-IgA)
Figure 1: Transfusion-Related Fatality Reports by Complication, FY2005 and FY2006

TRALI continues to be the leading cause of transfusion related fatality reported to CBER. Over the 2-year reporting period, TRALI represented 51% of confirmed transfusion related fatalities. The reports named a variety of different blood components; however, 61% of the TRALI fatalities were associated with receipt of Fresh Frozen Plasma or other plasma products (Figure 2). For comparison, in Calendar Year 2004, transfused plasma products accounted for approximately 19% of all transfused components, Apheresis Platelets – approximately 6%, and red blood cell-containing products – approximately 64%.3 Reporters identified donor antibodies to HLA Class I and/or Class II antigens in 56% of the TRALI cases. Occasionally (8 cases in FY2006), reporters were able to match donor antibody(ies) and the cognate antigen(s) in the recipient(s).
As seen in previous years, the fatalities occurred in male and female recipients of all ages.4
In June, 2001, after hearing a number of presentations on TRALI, the Blood Products Advisory Committee (BPAC) recommended that each facility investigate TRALI reactions.5 In October, 2001, CBER distributed to the transfusion community a “Dear Colleague” letter6 on TRALI. The intent of the letter was to raise awareness of this under-recognized and often misdiagnosed complication of transfusion which, if not appropriately and promptly treated, may lead to death. The letter included a description of the syndrome, recommendations for diagnosis, treatment, and follow-up, and recommended MedWatch reporting of non-fatal TRALI cases. Reports of TRALI-related fatalities began to increase following issuance of the letter, suggesting that it may have improved physician recognition of TRALI. However, there has not been a noticeable increase in MedWatch reports of non-fatal events from TRALI. We continue to encourage the use of the MedWatch report mechanism for non-fatal TRALI events.
On May 30, 2003, the National Heart Lung and Blood Institute (NHLBI) convened a working group on TRALI. The NHLBI Working Group defined TRALI as a new onset of acute lung injury (ALI) occurring within 6 hours after the transfusion of plasma-containing blood components.7 In April, 2004, the Canadian Consensus Conference Panel set forth additional criteria for defining TRALI and recommended that blood collection facilities assess the value and cost of TRALI interventions.8 , 9In recent publications, CBER staff have summarized the U.S. experience with TRALI.10 , 11
Figure 2: Reports of TRALI by Implicated Blood Product, FY2005 and FY2006
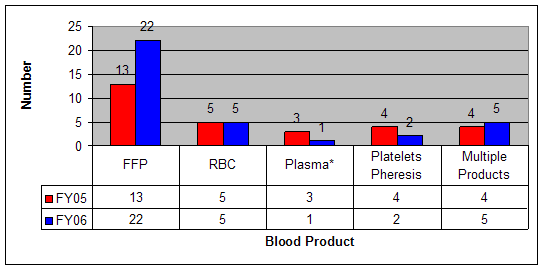
*FY2005: Includes 2 FP24 (Plasma frozen within 24 hours after collection) and 1 Liquid Plasma
FY2006: Includes 1 FP24
Fatalities due to red blood cell antibodies other than ABO continue to occur, due both to variability in detection methods and antibody characteristics. No single method is capable of detecting all clinically significant antibodies.12 , 13 In combined FY2005 and FY2006, reporters implicated non-ABO antibodies in 74% of the total fatal hemolytic transfusion reactions, with 29% of these attributed to multiple antibodies (Table 2).
Table 2: Hemolytic Transfusion Reactions by Implicated Antibody, FY2005 and FY2006
| Antibody | FY2005 | FY2006 | Total (FY05+FY06) | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Multiple Antibodies** | 6 | 27% | 4 | 33% | 10 | 29% |
| ABO | 6 | 27% | 3 | 25% | 9 | 26% |
| Other* | 3 | 14% | 0 | 0% | 3 | 9% |
| Jkb | 3 | 14% | 0 | 0% | 3 | 9% |
| Jka | 1 | 5% | 1 | 8% | 2 | 6% |
| Kell | 1 | 5% | 1 | 8% | 2 | 6% |
| Fya | 0 | 0% | 1 | 8% | 1 | 3% |
| Fyb | 0 | 0% | 1 | 8% | 1 | 3% |
| E | 1 | 5% | 0 | 0% | 1 | 3% |
| I | 1 | 5% | 0 | 0% | 1 | 3% |
| Jsa | 0 | 0% | 1 | 8% | 1 | 3% |
| Totals | 22 | 100% | 12 | 100% | 34 | 100% |
*Includes one report of non-immune hemolysis, one report of an unidentified antibody to a low incidence antigen, and one report of Cold Agglutinin Syndrome due to Mycoplasma pneumonia or Lymphoma.
**FY2005 antibody combinations included E+c, Fya +K, Fya +Jkb , E+I+A1, possible C+E+K, Wr a +warm autoantibody.
**FY2006 antibody combinations included E+c, S+K, Jkb +cold agglutinin, unidentified auto- and alloantibodies.
In FY2005, hemolytic transfusion reactions due to ABO-incompatible blood transfusions resulted in the following 6 fatalities:
In FY2006, errors in recipient identification at the time of transfusion preceded all 3 fatal ABO-incompatible transfusions.
On February 26, 2004, FDA published a final rule16 requiring the use of machine-readable information on blood and blood component container labels. The compliance date for the use of this machine-readable information was April 26, 2006. A main purpose of the rule was to help prevent transfusion errors in hospitals and health care settings. The FDA hopes to see progress in this area as more hospitals use machine-readable information from the time of patient sample collection through transfusion.
Bacteria and other microorganisms can be present in blood components collected from an asymptomatic infected donor. Additionally, venipuncture can introduce bacteria from skin flora, and collection bag defects or blood component processing may allow bacteria to enter the blood component. Proliferation of bacteria during component storage increases the risk of sepsis in transfusion recipients. Since the voluntary implementation of bacterial detection testing for platelet components in March 2004, we have noted a decrease in the number of reported fatalities associated with transfusion of bacterially contaminated apheresis platelets (Figure 3). There were no common contaminants reported between FY2005 and FY2006; however, Staphylococcus aureus and Escherichia coli accounted for the majority of the fatalities due to microbial infection (Table 3). Of note are two FY2006 cases of Babesia transmission following Red Blood Cell transfusions from donors who subsequently tested positive for Babesia microti.
Table 3: Microbial Infection by Implicated Organism, FY2005 and FY2006
| Organism | FY05 | FY06 | Total (FY05+FY06 | |||
|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | |
| Staphylococcus aureus | 3 | 37% | 0 | 0% | 3 | 20% |
| Escherichia coli | 0 | 0% | 3 | 43% | 3 | 20% |
| Serratia marcescens | 2 | 24% | 0 | 0% | 2 | 13% |
| Babesia microti | 0 | 0% | 2 | 29% | 2 | 13% |
| Staphylococcus lugdunensis | 1 | 13% | 0 | 0% | 1 | 7% |
| Staphylococcus epidermidis | 1 | 13% | 0 | 0% | 1 | 7% |
| Eubacterium limosum | 1 | 13% | 0 | 0% | 1 | 7% |
| Morganella morganii | 0 | 0% | 1 | 14% | 1 | 7% |
| Yersinia enterocolitica | 0 | 0% | 1 | 14% | 1 | 7% |
| Totals | 8 | 100% | 7 | 100% | 15 | 100% |
Figure 3: Microbial Infection by Implicated Blood Product, FY2005 and FY2006
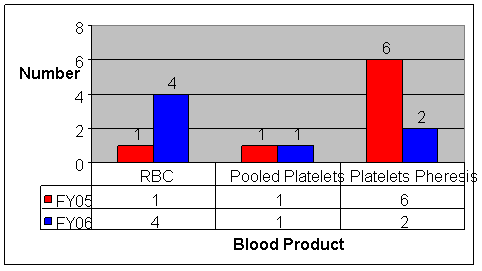
Red Blood Cells microorganisms: S. Marcescens (1), E. Coli (1), Y. Enterocolitica (1), B. Microti (2)
Pooled Platelets microorganisms: S. aureus (1), E. coli (1)
Platelets Pheresis microorganisms: S. aureus (2), S. marcescens (1), S. lugdunensis (1), S. epidermidis (1), E.limosum (1), E. coli (1), M. morganii (1)
After reviewing the initial fatality reports and the investigation documentation, we categorized a number of reported fatalities as “Not Transfusion Related.” The medical reviewers concluded that, while there was a temporal relationship between transfusion and subsequent death of the recipient, there was no evidence to support a causal relationship (Table 4). Thus, we did not include these reported fatalities in the analysis in Sections II.A through II.D (transfusion-related fatalities), above.
In these reported fatalities, the reporting facilities were unable to identify a specific complication of transfusion as the cause of death. Often, these patients had multiple co-morbidities, and after review of the investigation documentation, the medical reviewers could neither confirm nor rule out the transfusion as the cause of the fatality (Table 4). We did not include these reported fatalities in the analysis in Sections II.A through II.D (transfusion-related fatalities), above.
Table 4: Fatalities Not Related to Transfusion or Transfusion Not Ruled Out, FY2005 and FY2006
| FY05 | FY06 | Total (FY05+FY06) | |
|---|---|---|---|
| Not Transfusion-Related | 21 | 8 | 29 |
| Not Ruled Out | 14 | 10 | 24 |
| Totals | 35 | 18 | 53 |
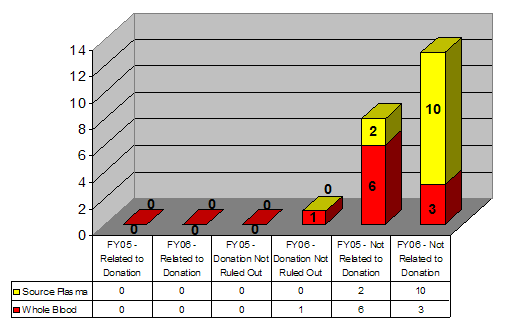
In FY2005, we received reports of 6 fatalities following allogeneic Whole Blood donation. In all 6 cases, the medical reviewers did not find a causal relationship between the Whole Blood donation and subsequent death of the donor. In FY2006, there were 4 reports of fatalities following Whole Blood donation, including 2 autologous donations. In three of the cases, the medical reviewers found no causal relationship between the donation and subsequent death of the donor. In the fourth case, an autologous donation, the medical reviewers could neither confirm nor rule out if the donor’s mild anemia prior to surgery contributed to the post-surgery fatality.
In FY2005 there were 2 reports of fatalities following Source Plasma donation and in FY 2006, there were 10 reports. For both FY2005 and FY2006, the medical reviewers concluded that, while there was a temporal link, there was no evidence to support a causal relationship between the Source Plasma donation and subsequent death of the donor.
Table 5: Post-donation fatalities reported in FY2005 and FY2006
| Donated Product | FY2005 | FY2006 | Total (FY05+FY06) |
|---|---|---|---|
| Source Plasma | 2 | 10 | 12 |
| Whole Blood | 6 | 4* | 10 |
| Totals | 8 | 14 | 22 |
*Includes 2 autologous donations
Figure 4: Post-Donation Fatality Reports, FY2005 and FY2006
Footnotes
1. Whitaker BI, Sullivan M. The 2005 Nationwide Blood Collection and Utilization Survey Report. Washington (DC): Department of Health and Human Services; 2006.
2. Guidance for Industry: Notifying FDA of Fatalities Related to Blood Collection or Transfusion, September, 2003.
3. Whitaker BI, op. cit. Tables 4-1 and 4-3.
4. Holness L, et al. Fatalities caused by TRALI. Transfus Med Rev 2004;18(3):184-8.
5. Blood Products Advisory Committee 69 th meeting, June 15, 2001.
6. Transfusion Related Acute Lung Injury, Dear Colleague Letter, October, 2001.
7. Toy P, Popovsky MA, Abraham E, et al. Transfusion-Related Acute Lung Injury: definition and review. Crit Care Med 2005;33:721-6.
8. Goldman M, Webert KE, Arnold DM. et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev 2005;19:2-31.
9. Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion 2004;44:1774-1789.
10. Knippen M, Transfusion Related Acute Lung Injury. Amer Jour Nurs 2006;106(6):61-64.
11. Holness LG, Epstein JS. International Forum: Measures to prevent TRALI. Vox Sang 2007; 92 258-277.
12. Merry AH, Thomson EE, et al. Quantitation of antibody binding to erythrocytes in LISS. Vox Sang 1984;47:125.
13. Yaskanin DD, Jakway JL, Ciaverella DJ. Red blood cell diluent composition is important for detection of some anti-E. Immunohematology 2000;16:142.
14. Pietersz RNI, Engelfriet CP, Reesink HW. International Forum: Transfusion of apheresis platelets and ABO groups. Vox Sang. 2005;88:207-221.
15. Fung MK, Downes KA, Shulman IA. Transfusion of Platelets Containing ABO-Incompatible Plasma. Arch Pathol Lab Med. 2007;131:909-915.
16. 69 Federal Register 9120, February 26, 2004. See 21 CFR 606.121(c)(13).


